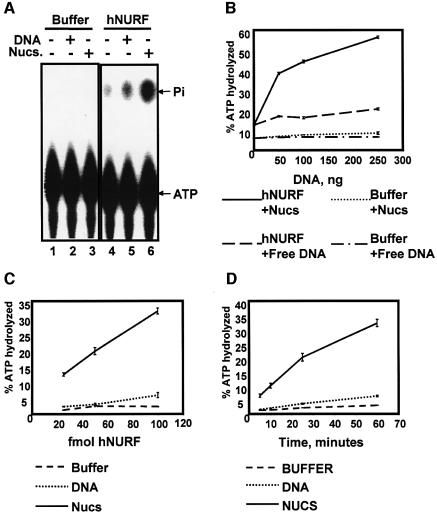
Fig. 5. hNURF exhibits intrinsic nucleosome-dependent ATPase activity. (A) hNURF exhibits DNA- and nucleosome-stimulated ATPase activity. ATPase activity assays performed on [γ-32P]ATP followed by separation of free phosphate (Pi) from ATP by PEI-cellulose TLC and autoradiography of TLC plates. hNURF activity is stimulated by supplementing the reaction with DNA (50 ng) and is potentiated further by nucleosomes (50 ng); compare lanes 4, 5 and 6. (B) hNURF activity responds to increasing amounts of nucleosomes. A 25 fmol concentration of hNURF or buffer was incubated with increasing amounts (0, 50, 100 or 250 ng) of either free DNA or free nucleosomes. Points represent the average of three reactions, with standard error as indicated. (C) hNURF exhibits a dose-dependent ATPase activity in the presence of nucleosomes. ATPase activity of three different hNURF concentrations (20, 50 and 100 fmol) with buffer, free DNA or nucleosomal DNA. Points represent the average of three reactions, with standard error as indicated. (D) Time course of hNURF ATPase activity. hNURF (100 fmol) was incubated with buffer, free DNA or nucleosomal DNA, and reactions were assayed for ATPase activity at the indicted times. Points represent the average of three reactions, with standard error as indicated.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.