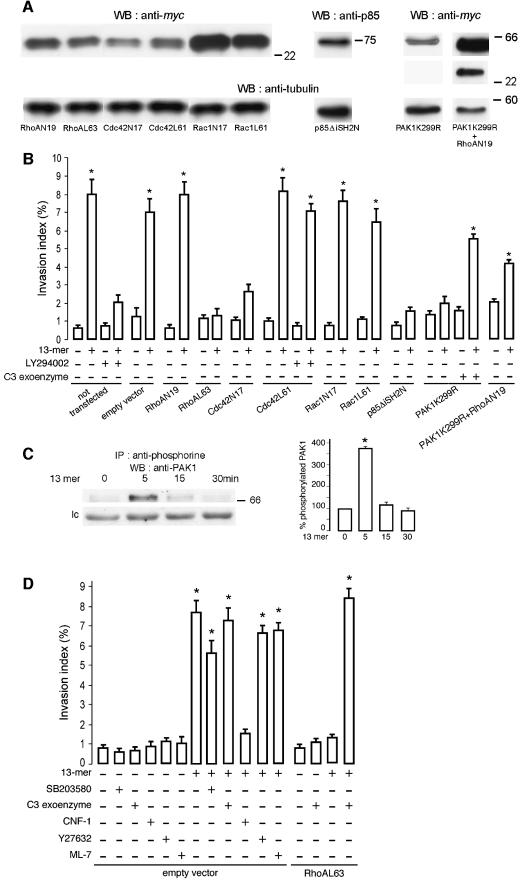
Fig. 5. Cdc42 and PI3K but not Rac1 are necessary for the 13mer peptide-mediated invasion. (A) Dominant-negative or constitutively active RhoA, Cdc42 or Rac1, dominant-negative PI3K or kinase-dead PAK1 or double kinase-dead PAK1 and dominant-negative RhoA expression in transiently transfected cells. Total lysates were immunoblotted with anti-myc or anti-p85 antibodies. Loading of equal amounts of protein was controlled by anti-α-tubulin immunoblotting. (B) HCT-8/E11 cells were transiently transfected with an empty vector or with dominant-negative RhoA (RhoAN19), Cdc42 (Cdc42N17) or Rac1 (Rac1N17), constitutively active RhoA (RhoAL63), Cdc42 (Cdc42L61) or Rac1 (Rac1L61), dominant-negative PI3K (p85ΔiSH2N), kinase-dead PAK1 (PAK1K299R) or kinase-dead PAK1+dominant-negative RhoA (PAK1K299R+RhoAN19) constructs. Twenty-four hours after transfection, cells were tested in the collagen invasion assay with (+) or without (–) the 13mer peptide (10–7 M). The PI3K inhibitor LY294002 (10–5 M) or the C3 exoenzyme (6.6 µg/ml) were added in selected assays. Invasion indices (%) were determined as in Figure 1A. *Significantly different from untreated (–) cells, transfected with an empty vector, at p < 0.005. (C) The 13mer peptide stimulates PAK1 serine- phosphorylation. Lysates of HCT-8/E11 cells, treated (for 0, 5, 15 or 30 min) with the 13mer peptide (10–7 M), were immunoprecipitated using an anti-phosphoserine antibody and immunoblotted with an anti-PAK1 antibody. A non-identified serine phosphorylated protein, crossreacting with the anti-PAK1 antibody, is presented as loading control (lc). Immunoblots were quantified as in Figure 4B (right panel), considering as 100% the phosphorylation level of untreated cells. (D) Cells were transiently transfected with an empty vector or with the constitutively active RhoA (RhoAL63) construct. Twenty-four hours after transfection, cells were incubated, on top of collagen type I gels, with (+) or without (–) the 13mer peptide (10–7 M) and/or SB203580 (1mg/ml), C3 exoenzyme (6.6 µg/ml), CNF1 (10–9 M), Y27632 (10–6 M) or ML-7 (5 × 10–6 M). Invasion indices (%) were determined as in Figure 1A. *Significantly different from untreated (–) cells, at p < 0.005.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.