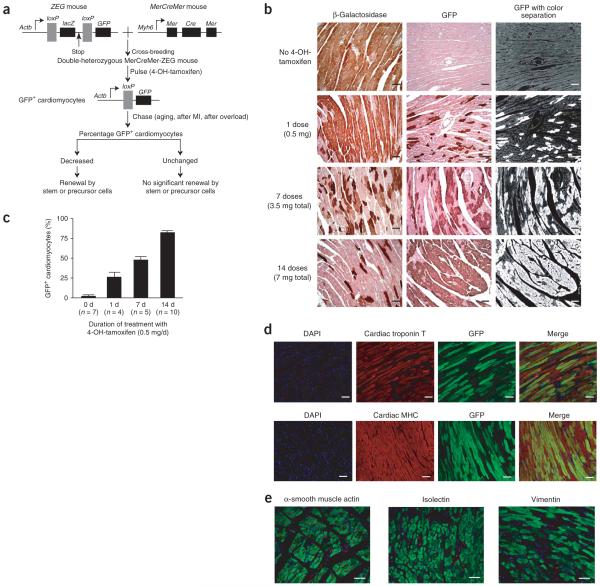
Figure 1.
Generation of MerCreMer-ZEG mice for genetic fate mapping of adult mammalian cardiomyocytes. (a) A dual reporter strain ZEG mouse was crossbred with the MerCreMer strain to create MerCreMer-ZEG double heterozygous mice. Upon the pulse of 4-OH-tamoxifen, the reporter switches from β-galactosidase (encoded by lacZ) to GFP in cardiomyocytes only. A reduction in the percentage of GFP+ cardiomyocytes during aging or following myocardial infarction (MI) or pressure overload might indicate that new cardiomyocytes have been generated by stem or progenitor cells. (b) Dose-dependent effects of the 4-OH-tamoxifen pulse on GFP labeling in MerCreMer-ZEG mouse myocardium. Representative immunohistochemistry images of β-galactosidase (left) or GFP (center) after different durations of daily 4-OH-tamoxifen, counterstained with hematoxylin. A computer-based color separation technique (right) was used to facilitate the counting of GFP-positive cardiomyocytes; the result is shown in c. (d) High-efficiency cardiomyocyte GFP labeling. We used immunofluorescence costaining to visualize nuclei (DAPI; blue), cardiomyocytes (cardiac troponin T or cardiac MHC; red), and GFP (green). The yellowish cells indicate cardiomyocytes labeled with GFP. (e) Labeling was specific for cardiomyocytes. Immunostaining was used to examine GFP expression in other cell types, including vascular smooth muscle cells (α-smooth muscle actin; red), endothelial cells (isolectin-B4, red), and fibroblasts (vimentin; red). Scale bars, 20 μm. Data shown as mean ± s.e.m.