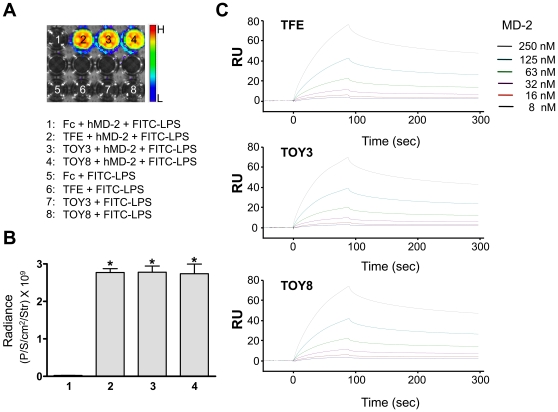
Figure 2. In vitro binding analysis reveals that LPS binds to TFE, TOY3, or TOY8 through MD-2 and BIAcore analysis of interaction between TFE, TOY3, or TOY8 and MD-2.
(A) 200 ng of Fc, TFE, TOY3, or TOY8 were coated onto 96-well plates. FITC-labeled LPS (10 µg/ml) was incubated in the presence or absence of MD-2 (1 µg/ml) in each indicated well for 2 hr. The fluorescence signal was measured by IVIS imaging. (B) Fluorescence was quantified and expressed as radiance (photon/sec/cm2/steradian). Bars represent means ± S.D. (n = 4). *, P<0.05 versus Fc+hMD-2+FITC-LPS (1). The x-axis numbering represents the number of each well in (A). (C) Sensorgrams for the association and dissociation of MD-2 on immobilized TFE, TOY3, or TOY8. One microgram of TFE, TOY3, or TOY8 was immobilized on a Sensor Chip CM5 (BIAcore) using N-hydroxysuccinimide (NHS) and 1-ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC) amine coupling reagent at approximately 2,000 resonance units (RU). As a control, BSA protein was immobilized on another portion of the same chip. Recombinant MD-2 proteins were then applied onto the immobilized TFE, TOY3, or TOY8 surfaces, and the amount captured was recorded in sensorgrams as RU. All samples were in running buffer to minimize bulk effects. The kinetic parameters of the binding interactions were calculated from the sensorgrams by nonlinear curve fitting using BIAEVALUATION software (BIAcore). RU represents resonance units.