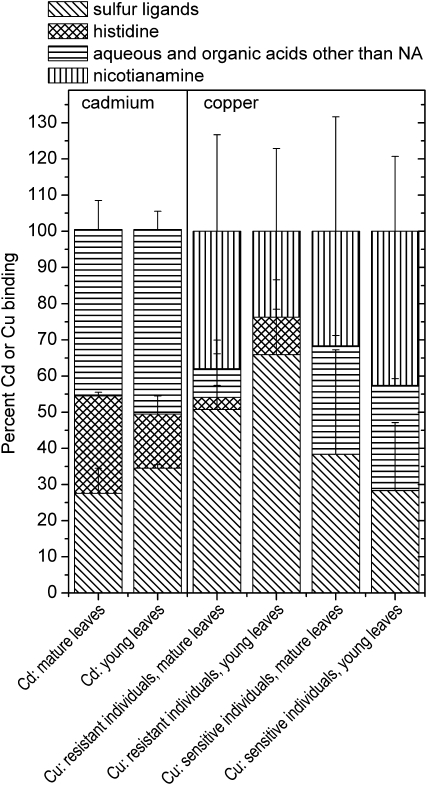
Figure 6.
Metal-, age-, and specimen-dependent differences in ligand environment. Right, Averaged samples of leaves from plants grown on 10 μm Zn2+ and 10 μm Cu2+: Cu-K edge (data from this study). Plant EXAFS spectra (k3 weighed) were fitted with a linear combination of the EXAFS spectra of the following model compounds: aqueous (CuSO4), Cu(II)-malate, Cu(II)-citrate, Cu(II)-Pro, Cu(II)-His, Cu(II)-NA, pH 4, Cu(II)-NA, pH 7, and Cu(I/II)-glutathione. Pro was never detected as a ligand, as was the aquo complex. The data of Cu(II)-malate and Cu(II)-citrate were averaged like the data of Cu(II)-NA at pH 4 and 7. Left, Averaged samples of mature leaves from plants grown on 10 μm Zn2+ and 100 μm Cd2+: Cd-K edge (data from Küpper et al., 2004). Plant x-ray absorption spectra were fitted with a linear combination of the EXAFS spectra of the following model compounds: aqueous (CdSO4), Cd(II)-malate, Cd(II)-citrate, Cd(II)-His, and Cd(II)-glutathione. The data of Cd(II)-malate, Cd(II)-citrate, and the aquo complex were averaged. Similar results as for Cd were obtained for Zn; see Küpper et al. (2004).