FIG. 7. Ccz1 and Mon1 physically interact.
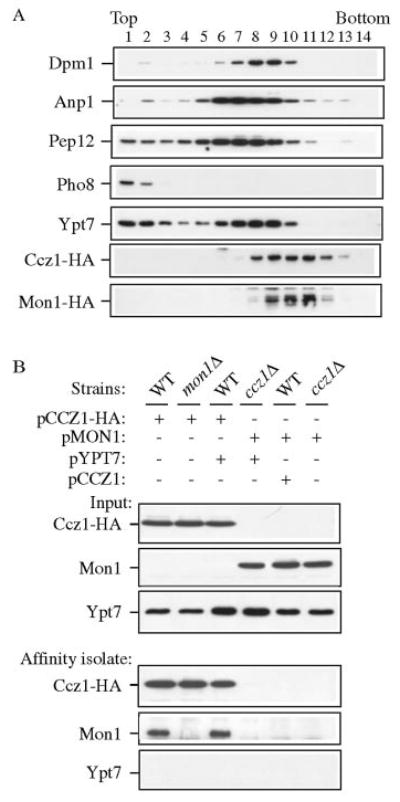
A, Ccz1 and Mon1 co-fractionated but were separated from endomembrane marker proteins by OptiPrep density gradients. The Mon1-HA strain (PSY35) expressing pCCZ1-HA(416) was analyzed by density gradient separation as described under “Experimental Procedures.” Fractions were subjected to immunoblot using antisera or antibodies to Dpm1 (ER), Anp1 (Golgi), Pep12 (endosome), Pho8 (vacuole), Ypt7, and HA. B, Ccz1-HA co-precipitates Mon1 by native immunoprecipitation. Wild type, ccz1Δ (CWY3), and mon1Δ (JSY1) strains were transformed with pCCZ1-HA(426), pMON1(426), and/or pYPT7(424), and were grown to mid-log phase followed by glass bead lysis in HEPES native immunoprecipitation buffer. An aliquot (10 μl) of lysate was used as the loading control. Lysates were incubated with anti-HA antibody and protein A-Sepharose as described under “Experimental Procedures” and subjected to immunoblot against HA, Mon1, and Ypt7.
