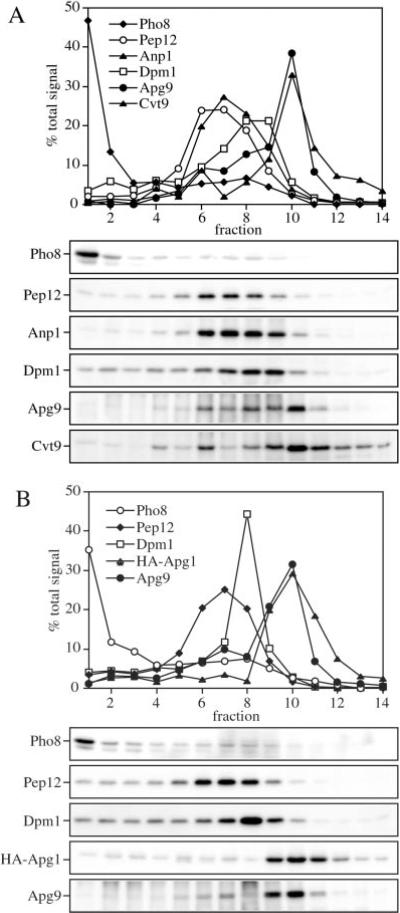
Fig. 5. Cvt9, Apg9, and Apg1 co-localize with each other but not with other endomembrane markers by density gradient separation.
Subcellular co-localization of Apg9 and Cvt9 (A) and HA-Apg1 and Apg9 (B) by OptiPrep density gradients. The wild type strain (SEY6210) was co-transformed with the multicopy APG9 plasmid (pAPG9(426)) and the copper-inducible CVT9 plasmid (pCuCVT9(416)) (A) or a plasmid expressing an HA epitope-tagged Apg1 (pHAAPG1(423)) the multicopy APG9 plasmid (pAPG9(426)) (B). Cells were grown to midlog stage in SMD, and those in A incubated with 50 μm CuSO4 for 2 h to induce Cvt9 expression. The cells were converted to spheroplasts and lysed in PS200 buffer as described under “Experimental Procedures.” A total membrane fraction was isolated by centrifugation at 100,000 × g for 20 min and loaded to the top of a 10-ml OptiPrep linear gradient (10–55%). Following centrifugation at 100,000 × g for 12 h at 4 °C, 14 fractions were collected and analyzed by immunoblots with antiserum or antibodies to Pho8 (vacuole), Pep12 (endosome), Anp1 (cis-Golgi), Dpm1 (ER), Cvt9, Apg9, and the HA epitope (Apg1) as indicated. Indirect chemiluminescent detection and quantification of relative protein concentrations were performed with the Bio-Rad Fluor-S Max Imager. Cvt9, Apg1, and Apg9 co-localize to a dense part of the gradient and are separated from known endomembrane markers.