Abstract
Background and Objectives
In the Rh blood group system, partial D, C, and e antigens are well-known, but a partial c antigen resulting in the production of alloanti-c in a c+ individual is rare. One example of an alloanti-c in a c+ person was an anti-Rh26, which can appear as anti-c, and another was an alloanti-c in a c+ person with a presumed R1r phenotype. The finding of an apparent alloanti-c in a transfused c+ patient initiated this investigation.
Materials and methods
Hemagglutination tests, DNA extraction, PCR-based assays (PCR-RFLP, AS-PCR), reticulocyte mRNA extraction, RT-PCR, and sequencing were performed by standard procedures.
Results
Plasma from a 64 year-old African American woman with a wound infection following a mastectomy, contained anti-E, anti-S, anti-K, anti-Fya and anti-Jkb, reacting by the indirect antiglobulin test (IAT). In addition, the patient’s plasma gave reactions that were consistent with an anti-c, while her pretransfusion RBCs typed c+ with some anti-c reagents. These results are consistent with a partial c antigen. The patient’s RBCs also typed V+WVS− and JAL+. Analyses of DNA and Rh-transcripts from this patient showed the presence of the following genes: RHD*D, RHD*DAU0, RHCE*Ce, and RHCE*ceS(340).
Conclusion
The nucleotide 340C>T change in RHCE exon 3 (predicted to encode 114Trp) of the RHCE*ceS(340) allele is associated with a JAL+ phenotype and the altered expression of the c, V, and VS antigens. This alteration in the c antigen allowed the patient to make an alloanti-c. This case reveals that the RHCE*ceS(340) allele encodes a partial c antigen.
Introduction
The Rh blood group system is the most polymorphic of the human blood group systems [1]. In this system, partial D, C, and e antigens are well-known, but a partial c antigen that allows the production of alloanti-c in a c+ individual is rare. Only two examples have been reported. One, an alloanti-c in a c+ (presumed phenotype R1r) person, was reported by Moulds and coworkers [2]. The other was anti-Rh26, which can appear as anti-c, and has been made by a Rh26−, c− person [3] and also by a RH26−, c+ person [4]. Molecular studies have shown that Rh26 is antithetical to the low-prevalence antigen LOCR and serological studies have shown that the LOCR+ phenotype encodes altered (weakened) expression of c [5].
Other altered c antigens have been reported, e.g., (c)(e)Be(a+), (c)(e)JAL+, (c)(E), and (c)(e) [1,6] but to date, people with these altered c antigens, have not been reported to make alloanti-c. We describe here serological testing on blood from a c+ patient whose serum contained an alloanti-c. Our findings reveal that the patient’s red blood cells (RBCs) are JAL-positive, that she is heterozygous for the rare RHCE*ceS(340) allele, and that this allele encodes a partial c antigen.
Case Study
The patient, a 64 year-old African American woman who had a wound infection following a mastectomy, had a previously identified anti-E. She was transfused with 3 units of E-negative packed RBCs two weeks prior to the investigation described here. Following this transfusion, the patient’s plasma reacted with all screening cells and all reagent red cells on an identification panel; the autocontrol was negative. A sample was submitted for identification of multiple antibodies or an alloantibody to a high-prevalence antigen. The referring hospital requested two units of packed RBCs for transfusion. The patient suffered renal failure and was treated with dialysis. Later, she died and no further samples could be obtained.
Material and Methods
Hemagglutination
Hemagglutination was performed by standard procedures using various media. Elution was performed using ELU-kit II from Gamma Biologicals, Inc. (Houston, TX). Anti-c reagents and reagent RBCs were purchased from Immucor-Gamma (Norcross, GA) and Ortho Clinical Diagnostics (Raritan, NJ) or were from our in-house libraries.
Molecular analysis
Genomic DNA extraction, amplification, and sequencing
Genomic DNA was isolated with a DNA extraction Kit (QIAamp DNA Blood Mini Kit, QIAGEN, Inc., Valencia, CA) from WBCs collected in EDTA and from RBC droplets frozen in liquid nitrogen. Polymerase chain reaction (PCR) amplification was performed with RH-specific primers (Invitrogen, Carlsbad, CA.), as previously described [7] and the products were analyzed by PCR-RFLP or direct sequencing by the University of Pennsylvania or the New York Blood Center DNA Sequencing Facility.
RNA extraction and Rh-cDNA cloning and sequencing
RNA was isolated from the RBCs of the patient (QIAzol, QIAGEN, Inc., Valencia, CA). Reverse transcription was carried out with Superscript II and random hexamers and oligo(dT) primer, according to the manufacturer’s instructions (Superscript First Strand Synthesis System, Invitrogen, Carlsbad, CA). PCR amplification was carried out for 35 cycles with primers complementary to the 5′ and 3′ regions of RHCE and RHD cDNAs. PCR products were checked for purity on agarose gels, recovered with gel isolation (MinElute PCR purification, QIAGEN), and cloned into TOPO II (Invitrogen) for sequencing. Sequences were aligned, and protein sequence comparisons were performed with CLUSTALX [7].
Results
Hemagglutination
The patient’s pre-transfusion RBCs initially typed group O; D+ C+ E−c+W e+ (most probable genotype R1R0); M+, N+, S−, s+; P1+; K− Fy(a−b−); and Jk(a+b−). Five phenotype-matched RBC samples [c+, E−, S−, K−, Fy(a−b−), Jk(b−)], were strongly (4+) incompatible by the PEG-antiglobulin technique. The patient’s RBCs were tested with numerous antibodies to high-prevalence antigens but all were reactive. An allogeneic adsorption was performed with phenotypically similar D+C−E−c+e+ (R0) RBCs and an eluate prepared from these adsorbing RBCs showed alloanti-c reactivity. Subsequently, additional phenotype-matched reagent RBCs that were also c− were tested, and found to be non-reactive with the patient’s plasma. Using selected c-negative RBCs, it was possible to identify anti-E, anti-S, anti-K, anti-Fya and anti-Jkb, all reacting by the indirect antiglobulin test (IAT).
The presence of an apparent alloanti-c in the plasma of a patient with c+ RBCs is consistent with a partial c antigen. Thus, the patient’s RBCs were tested for the presence of antigens known to be associated with altered C and c antigens. The patient’s RBCs were shown to lack the following antigens: f, Goa, Rh32, Bea, Tar, FPTT, and JAHK. The patient’s RBCs typed JAL+, V+W, and VS−(n = 1 monospecific anti-VS). As the f type was unexpected, the typing was repeated with a total of six anti-f reagents; none agglutinated the patient’s RBCs. The eluate containing anti-c was tested against three examples of D− – RBC samples, a Dc− RBC sample, and four c+ JAL+ RBC samples. Three of the JAL+ RBC samples have a single dose expression of the JAL antigen and one sample has a double dose expression [7] (Table 1). The trace reactivity obtained with one R0 with a double dose expression of JAL and one D− - was considered to be due to background “noise”.
Table 1.
Tests with an eluate prepared from R0 phenotypically-matched RBCs against selected RBC samples
| Phenotype of RBC sample | IAT |
|---|---|
| R2R0 JAL+ single dose | 1+W |
| R0R0 JAL+ single dose | 1+ |
| R1R2 JAL+ single dose | 1+ |
| R0R0 JAL+ double dose | Trace |
| D− – | 0 |
| D− – | Trace |
| D− – | 0 |
| Dc− | 1+ |
| R1R1 | 0 |
| R2R2 | 3+W |
The patient’s RBCs were tested with multiple examples of polyclonal and monoclonal anti-c reagents (Table 2). The patient’s RBCs were non-reactive with some reagents in direct agglutination tests but all were reactive in the IAT, albeit more weakly than C+c+ control RBCs. Of three commercial monoclonal anti-c reagents, one (Gamma-Clone, which contains clone 951) was nonreactive, one was weakly reactive (Immucor, which contains clone MS33), and one was strongly reactive (Ortho Bioclone, which contains clone MS42).
Table 2.
Typing the patient’s RBCs with multiple anti-c reagents
| Anti-c reagents (n=22) | Patient’s RBCs | C+c+ control | ||
|---|---|---|---|---|
| DA | IAT | DA | IAT | |
| Polyclonal (n = 13) | ||||
| BCA, Dade, Immucor, Ortho | 0 | 2+ | 4+ | 3+ |
| Gamma, Immucor | 3+ | NT | 4+ | 3+ |
| BCA (n=1), Immucor (n=5) | ± | 2+ to 3+W | 4+ | 3+ |
| Single donor | NT | 0 | NT | 2+ |
| Monoclonal (n = 9) | ||||
| GammaClone 951 (n=2)* | 0 | NT | 4+ | NT |
| Immucor, Clone MS33 (n =2)* | 1+ | NT | 4+ | NT |
| Ortho, BioClone MS42 (n=2)* | 3+ | NT | 4+ | NT |
| RaE11 single clone (n=2)* | 0 | 1+/2+ | 0 | 4+ |
| BS240 single clone | NT | 3+ | NT | 4+ |
DA = direct agglutination
IAT = indirect antiglobulin test
NT = not tested
= different lot numbers
Collectively, our results show that the C+c+JAL+ RBCs from this patient have a variant c antigen that allowed the production of an anti-c.
DNA analysis
Analyses of genomic DNA showed the presence of RHCE*Ce and RHCE*ce alleles with heterozygosity for nucleotide 733C/G (245Leu/Val) and homozygosity for the wild type nucleotide1006G (Gly336), which predicts expression of V and VS antigens. Rh transcripts from this patient showed the presence of the following alleles: RHD*D, RHD*DAU0, RHCE*Ce, and RHCE*ceS(340). This is described in detail as Sample 5 in Westhoff et al [7]. Both RHCE transcripts encoded the wild type nucleotide 286G (96Gly), showing that the patient does not have an allele encoding the Rh26− LOCR+ phenotype.
Discussion
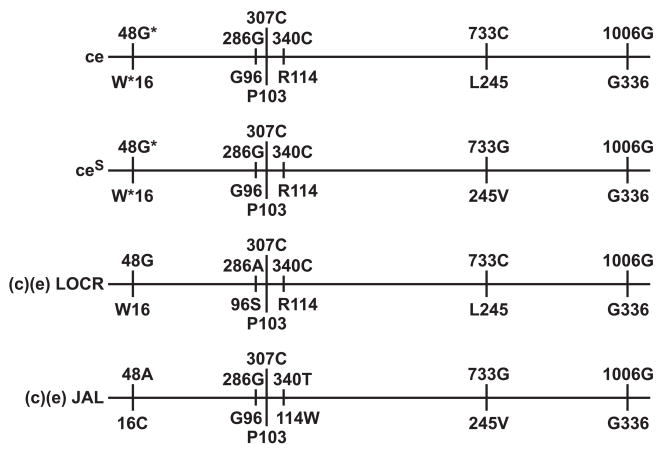
The RHCE*ceS(340) allele has a nucleotide 340C>T change in exon 3 of RHCE. This is predicted to encode 114Trp in place of Arg and is associated with a JAL+ phenotype [7]. The fact that the altered c antigen was present in single dose (in trans to C) in the patient described here, reveals that the RHCE*ceS(340) allele first reported by Noizat-Pirenne, et al., [8] encodes a partial c antigen. Furthermore, the alteration of the c antigen associated with the Arg114Trp amino acid change is such that the patient made an alloanti-c. The RHCE*ceS(340) allele not only encodes an altered expression of the c antigen (103 Pro) but, previously has been shown to also express an altered e antigen [8]. Noizat-Pirenne, and co-workers [8] reported that people whose RBCs had the RHCE*ceS(340) haplotype have made anti-e and anti-Ce. Despite the presence of the VS-encoding nucleotide [733G (245Val)], RBCs with this haplotype have weakened expression of the V antigen, and extremely weak expression of the VS antigen, which as in the case reported here can appear as VS-negative. The antigen profile of JAL+ RBCs is presented in detail as Sample 5 in Lomas-Francis, et al [9]. Figure 1 depicts the relevant nucleotides and amino acids associated with the (c)(e) JAL+ phenotype compared to some other ce haplotypes.
Figure 1. Nucleotides and amino acids associated with c+ e+ JAL+ compared to c+ e+ JAL−.
Alignment showing the critical positions of nucleotide changes (above the line) and amino acid changes (below the line) in relevant RHCE alleles
* = usually G and Trp (W) but can be A and Cys (C), especially in Blacks.
In summary, this case report illustrates that persons with the rare partial c phenotype encoded by the RHCE*ceS(340) allele readily produce an alloanti-c. We recommend that individuals whose RBCs are c+ and whose plasma contains anti-c are investigated to determine if the RBCs are JAL+ or, given the scarcity of anti-JAL, be analyzed for the presence of the RHCE*ceS(340) allele. If the RBCs are C+ then monoclonal anti-c can be used to indicate the presence of the ceS(340) haplotype: in direct agglutination tests, clone 951 (Gamma-Clone) is non reactive, while clone MS42 (Ortho Bioclone) is strongly reactive.
Acknowledgments
The work was funded in part by NIH grant NIH-NHLBI R01 HL091030 (MER).
We thank Denden Alcantara for some serological testing and Robert Ratner for help in preparing this manuscript.
References
- 1.Reid ME, Lomas-Francis C. Blood Group Antigen FactsBook. 2. San Diego: Academic Press; 2004. [Google Scholar]
- 2.Moulds JJ, Case J, Anderson TD, Cooper ES. The first example of allo-anti-c produced by a c-positive individual. Recent Advances in Haematology, Immunology and Blood Transfusion: Proceedings of the Plenary Sessions of the Joint Meeting of the 19th Congress of the International Society of Haematology and the 17th Congress of the International Society of Blood Transfusion; Budapest. August 1982; John Wiley & Sons; 1983. [Google Scholar]
- 3.Huestis DW, Catino ML, Busch S. A “New” Rh antibody (anti-Rh 26) which detects a factor usually accompanying hr’. Transfusion. 1964;4:414–418. doi: 10.1111/j.1537-2995.1964.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 4.Faas BHW, Ligthart PC, Lomas-Francis C, Overbeeke MAM, von dem Borne AEG, van der Schoot CE. Involvement of Gly96 in the formation of the Rh26 epitope. Transfusion. 1997;37:1123–1130. doi: 10.1046/j.1537-2995.1997.37111298088040.x. [DOI] [PubMed] [Google Scholar]
- 5.Coghlan G, Moulds M, Nylen E, Zelinski T. Molecular basis of the LOCR (Rh55) antigen. Transfusion. 2006;46:1689–1692. doi: 10.1111/j.1537-2995.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- 6.Lomas C, Poole J, Salaru N, Redman M, Kirkley K, Moulds M, McCreary J, Nicholson GS, Hustinx H, Green C. A low-incidence red cell antigen JAL associated with two unusual Rh gene complexes. Vox Sang. 1990;59:39–43. doi: 10.1111/j.1423-0410.1990.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 7.Westhoff C, Vege S, Wylie D, Lomas-Francis C, Hue-Roye K, Reid ME. The JAL Antigen (RH48) is the result of a change in RHCE that encodes Arg114Trp. Transfusion. 2008 doi: 10.1111/j.1537-2995.2008.02034.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noizat-Pirenne F, Lee K, Le Pennec P-Y, Simon P, Kazup P, Bachir D, Rousaud A-M, Roussel M, Juszczak G, Menanteau C, Rouger P, Cartron J-P, Ansart-Pirenne H. Rare RHCE phenotypes in black individuals of Afro-Caribbean origin: Identification and transfusion safety. Blood. 2002;100:4223–4231. doi: 10.1182/blood-2002-01-0229. [DOI] [PubMed] [Google Scholar]
- 9.Lomas-Francis C, Reid ME, Westhoff C, Alcantara D, Nickle P, Uehlinger J. JAL (RH48) blood group antigen: Serological observations. Transfusion. 2008 doi: 10.1111/j.1537-2995.2008.02025.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]