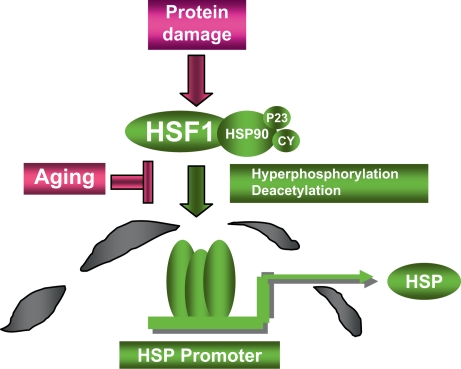
Fig. 4.
HSF1 regulation by damaged proteins. Damaged proteins may accumulate in cells due to exposure to proteotoxins or decline in protein degradation pathways. Such damaged proteins cause the release of HSF1 from inert cytoplasmic complexes containing Hsp90 and its co-chaperones including p23 and cyclophilins (CY), trimerization, migration to the nucleus and binding to HSP gene promoters. Full HSF1 activation involves the triggering of a complex network of posttranslational modifications that lead to hyperphosphorylation and deacetylation of key residues. Loss of HSF1 inducibility in aging may involve alterations in HSF1-chaperone complexes or in signal transduction pathways upstream of enzymes that regulate hyperphosphorylation or deacetylation.