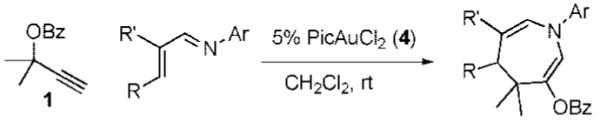
Table 2.
[4 + 3]-Cycloaddition of α,β-Unsaturated Imines
 | |||
|---|---|---|---|
| entry | imine | azepine | Yield |
| 1 | 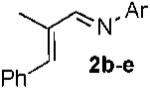 |
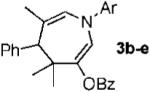 |
b Ar = 4-HO-2,6-Me2-C6H2 87% |
| 2 | c Ar = 2,6-Me2-C6H3 80% | ||
| 3 | d Ar = 2,3-Me2-C6H3 88% | ||
| 4 | e Ar = 4-MeO-C6H4 65% | ||
| 5 | f Ar = 4-F-C6H4 55% | ||
| 6 | 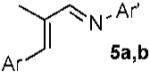 |
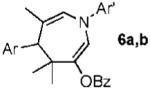 |
a Ar = 4-NO2C6H4 70% |
| 7 | b Ar = 4-MeOC6H4 80% | ||
| 8 | 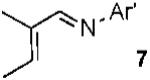 |
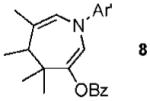 |
65% |
| 9 | 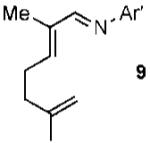 |
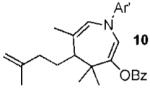 |
62% |
| 10 | 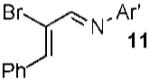 |
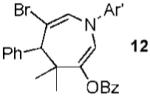 |
63% |
Conditions: 1.3 equiv of 1, 5% 4, CH2 Cl2, rt. Ar’ = 4-HO-2,6-Me2-C6H2.
