Table 3.
Diastereoselective Transformations of Propargyl Esters
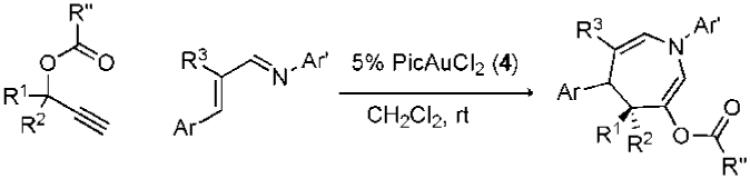 | ||||
|---|---|---|---|---|
| entry | propargyl ester | imine | azepine | yield (dr)a |
| 1 | 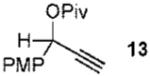 |
5a | 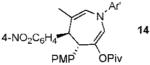 |
99% (> 20:1) |
| 2 | 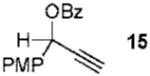 |
11 | 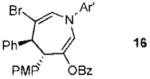 |
73% (> 20:1) |
| 3 | 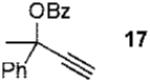 |
2b | 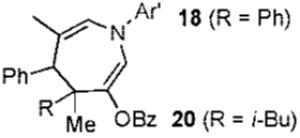 |
73% (3.3:1) |
| 4 | 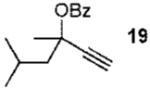 |
2b | 83% (1.4:1) | |
| 5 | 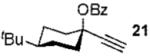 |
2b | 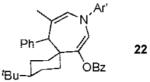 |
58%b (2.5:1) |
Conditions: 1.3 equiv of propargyl ester, 5% 4, CH2Cl2, rt.
Conditions: 2 equiv of propargyl ester, 10% 4, dichloromethane, 60 °C. Ar’ = 4-HO-2,6-Me2-C6H2.
