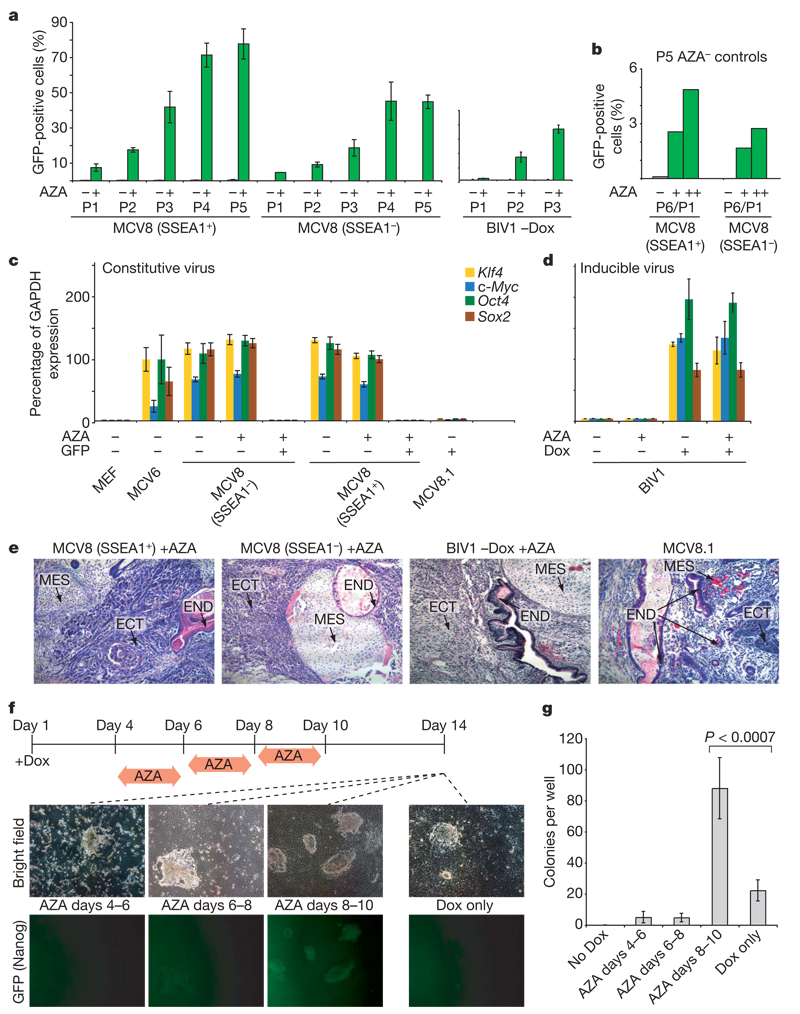
Figure 4. Inhibition of Dnmt1 accelerates the transition to pluripotency.
a, MCV8 (sorted by FACS using a SSEA1-specific antibody) and BIV1 (−Dox) were either exposed to AZA for 48h (green) or kept in regular embryonic stem cell medium (grey). The number of Oct4–GFP-positive cells was analysed over multiple passages (P) by FACS. b, Untreated MCV8 control cells from passage 5 were subsequently subjected to AZA treatment for 48 h (+) or 120h (++), and resulting Oct4–GFP-positive cells were counted after one passage. P6/P1, total passage 6/passage 1 after AZA treatment. c, AZA treatment does not influence retroviral expression levels. d, AZA treatment has no influence on lentiviral expression in uninduced or induced BIV1 cells. e, Pluripotencyof all AZA-treated lines and MCV8.1 was demonstrated by teratoma formation. ECT, ectoderm; END, endoderm; MES, mesoderm. f, Overall efficiency of AZA treatment. Nanog–GFP MEFs were plated on 6-well plates (4 wells per time point with Dox, and 2 wells without). Cells were treated with AZA during one of the indicated intervals. On day 14, colony formation was analysed by fluorescence microscopy (representative panels are shown). g, Number of alkaline-phosphatase-positive, embryonic-stem-cell-like colonies obtained from each treatment. AZA treatment during days 8–10 resulted in a ~4-fold increase in efficiency over untreated controls. For a, c, d and g, error bars show standard deviations (n = 2, 2, 2 and 4, respectively).