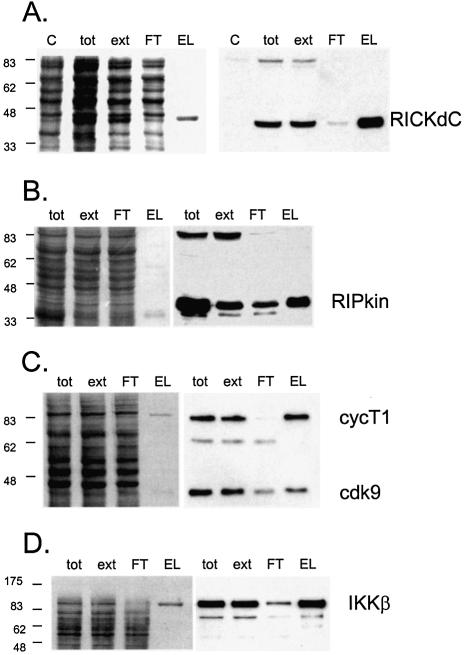
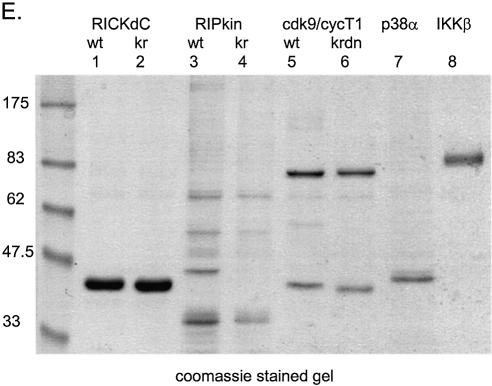
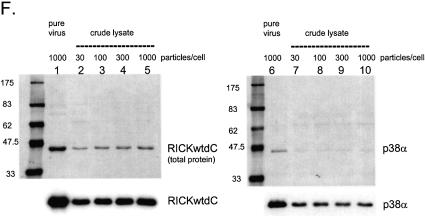
Figure 2.
Expression and purification of several protein kinases. Recombinant adenoviruses directing the expression of the indicated protein kinases were used to infect 293 cells at 1000 virus particles/cell. At 48 h post-infection the cells were harvested, washed twice with HBS (150 mM NaCl, 20 mM HEPES, pH 7.5), lysed, extracted and purified by streptactin–macropore as described in Materials and Methods. Aliquots of the total material before extraction (tot), the extract (ext), unbound material after expsoure to resin (FT) and the material eluted with desthiobiotin (EL) were mixed with SDS application buffer, heated at 99°C for 10 min, sonicated for 5 min in a sonicating water bath and 20 µl was loaded per well on a 10% acrylamide SDS gel. After resolution, the gel was transferred to nitrocellulose, stained with Ponceau S (A–D, left panels) or blocked with NET/gelatin and avidin, probed with streptactin–horseradish peroxidase (IBA) and visualized by ECL (A–D, right panels). (E) Equal aliquots of purified proteins were resolved on a 10% acrylamide SDS gel and visualized by staining with colloidal Coomassie blue (Sigma). (F) Comparison of pure virus to crude lysate inocula. 293 cells were infected with either 1000 particles/cell of CsCl-purified adenovirus encoding RICKdCst or p38αST or with crude infected cell lysates of cells infected with the same adenoviruses, at 30, 100, 300 or 1000 particles/cell. At 48 h post-infection, cells were harvested, lysed and the strep-tagged protein purified as described above. Equal volumes of each of the eluate fractions were resolved on a 10% acrylamide SDS gel, transferred to nitrocellulose, stained for total protein with Ponceau S (upper panels) and then probed with streptactin–horseradish peroxidase (IBA) and visualized by ECL (lower panels).