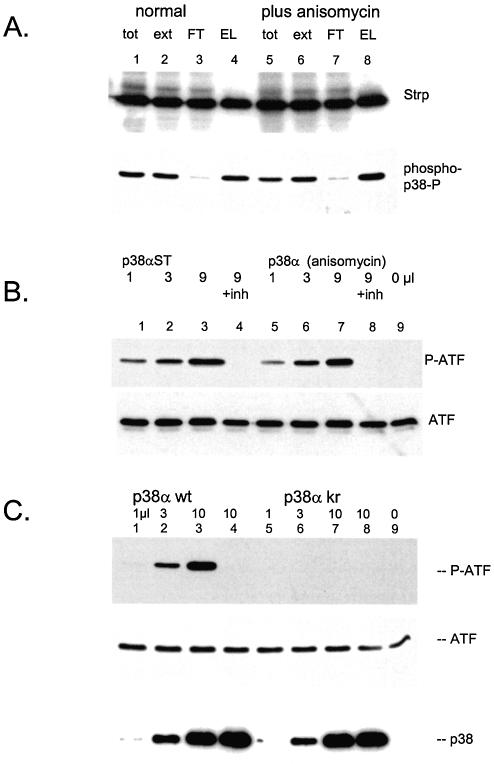
Figure 5.
p38α is generated in an active form. (A) Adenovirus directing a strep-tagged p38α was used to infect 293 cells. One portion of cells was treated with anisomycin for 30 min before harvest. Lysate preparation and purification by streptactin–macropore was performed as described above; aliquots were resolved on 10% acrylamide SDS gels, transferrred to nitrocellulose, blocked with NET/gelatin and avidin, probed with streptactin–horseradish peroxidase (top panel) or probed with anti-phospho-p38 (NEB) (bottom panel). (B) The indicated quantities of p38α purified from either control cells (lanes 1–4) or anisomycin stimulated cells (lanes 5–8) were incubated with ATF2 as described in Materials and Methods. Samples for lane 4 and 8 included 5 µM SB203580. The reactions were resolved on 10% acrylamide SDS gels, transferred to nitrocellulose blocked with NET/gelatin probed with anti-phospho-ATF (top panel) or antisera against total ATF (bottom panel). (C) The indicated quantities of purified p38α wild type (lanes 1–4) or kinase inactive p38α (lanes 5–8) were incubated with ATF2 as described in Materials and Methods. The reactions for lane 4 and 8 included 5 µM SB203580. The reactions were resolved on 10% acrylamide SDS gels, transferred to nitrocellulose and probed with anti-phospho-ATF2 antisera (top panel), anti-ATF2 sera (middle panel) or streptactin–horseradish peroxidase to visualize p38α (bottom panel).