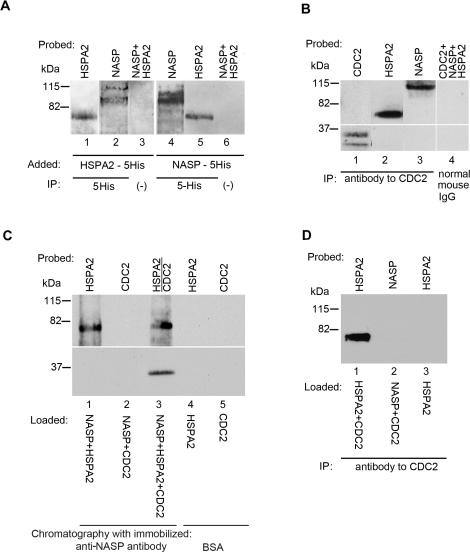
FIG. 2.
tNASP-HSPA2 binding in the nuclear fraction of mouse spermatogenic cells. A) Lanes 1 and 2: HSPA2-5His was added to a mouse germ cell nuclear fraction and immunoprecipitated (IP) with anti-5His antibody. Both recombinant HSPA2 (lane 1) and endogenous (nonrecombinant) tNASP (lane 2) were precipitated. In control (lane 3) without anti-5His antibody, neither HSPA2 nor tNASP was immunoprecipitated. Lanes 4 and 5: tNASP-5His was added to a mouse germ cell nuclear fraction and immunoprecipitated with anti-5His antibody. Both recombinant tNASP (lane 4) and endogenous (nonrecombinant) HSPA2 (lane 5) were precipitated. In control (lane 6) without anti-5His antibody, neither HSPA2 nor tNASP was immunoprecipitated. B) Immunoprecipitation of CDC2 (lane 1), HSPA2 (lane 2), and tNASP (lane 3) from the nuclear fraction of mouse spermatogenic cells by anti-CDC2 antibody. Control immunoprecipitation with normal mouse IgG demonstrated that CDC2, tNASP, and HSPA2 (lane 4) did not bind nonspecifically. C) Affinity chromatography with Aminolink-coupled recombinant tNASP bound recombinant HSPA2 (lane 1) but not recombinant CDC2 (lane 2). When HSPA2 and CDC2 were preincubated before addition to the tNASP affinity column, the complex was bound and eluted (lane 3). Control affinity chromatography with Aminolink-coupled bovine serum albumin demonstrated that recombinant HSPA2 (lane 4) and recombinant CDC2 (lane 5) did not bind nonspecifically to Aminolink. D) Anti-CDC2 antibody immunoprecipitates recombinant HSPA2 premixed with recombinant CDC2 (lane 1); however, it does not immunoprecipitate recombinant tNASP premixed with recombinant CDC2 (lane 2). The control (lane 3) shows that anti-CDC2 does not immunoprecipitate recombinant HSPA2.