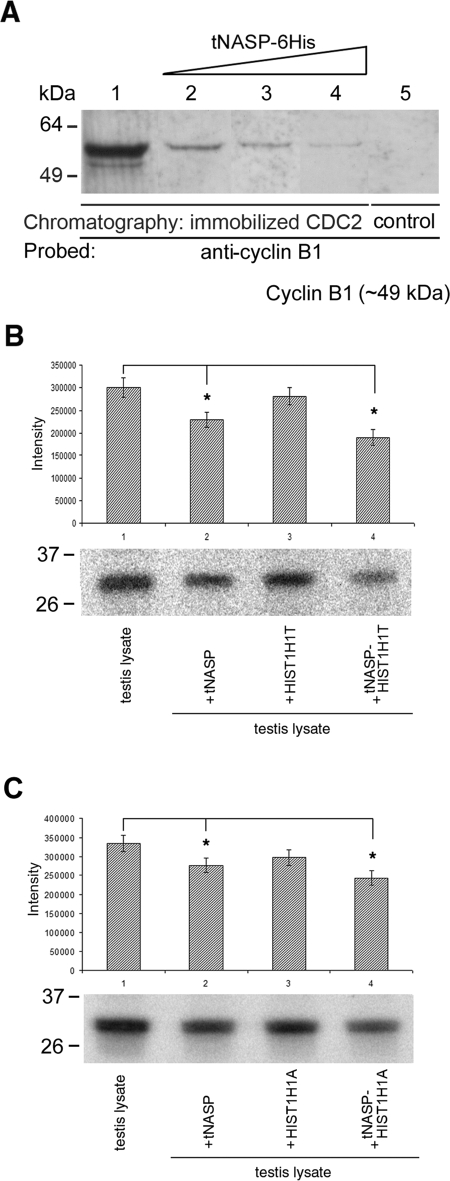
FIG. 3.
A) The presence of increasing amounts of tNASP decreases binding between CDC2 and cyclin B1 in nuclear fractions from mouse spermatogenic cells. Eluates from a CDC2 affinity column loaded with a nuclear fraction from mouse spermatogenic cells only (lane 1) or a nuclear fraction preincubated with increasing amounts of recombinant tNASP at 5 μM (lane 2), 10 μM (lane 3), and 25 μM (lane 4) show a decrease in cyclin B1 binding. The control using affinity beads without CDC2 cross-linked shows no cyclin B1 binding (lane 5). B and C) PhosphorImager detection of 32P-labeled histone indicating that CDC2/cyclin B1 kinase activity decreases in the presence of tNASP and H1 histones. B) tNASP (lane 2), HIST1H1T (lane 3), or tNASP + HIST1H1T (lane 4) was added to mouse testis lysates, and CDC2/cyclin B1 complexes were immunoprecipitated using anti-cyclin B1 antibody. After immunoprecipitation, the kinase activity was determined as a measure of the presence of 32P-labeled histone. The control (lane 1) consisted of a testis lysate alone. *Significant differences in the kinase activity: control vs. tNASP (P < 0.01); control vs. tNASP-HIST1H1T (P < 0.003); and tNASP vs. tNASP-HIST1H1T (P < 0.03). Data are represented as mean ± SD. C) Experiments identical to B were performed using histone HIST1H1A instead of HIST1H1T. Lane 1: control (testis lysate alone). tNASP (lane 2), HIST1H1A (lane 3), or tNASP + HIST1H1A (lane 4) was added to mouse testis lysates, and CDC2/cyclin B1 complexes were immunoprecipitated using anti-cyclin B1 antibody. After immunoprecipitation, the kinase activity was determined as a measure of the presence of 32P-labeled histone. *Significant differences in the kinase activity: control vs. tNASP (P < 0.02); control vs. tNASP-HIST1H1A (P < 0.006); and tNASP vs. tNASP-HIST1H1T (P < 0.05). Data are represented as mean ± SD.