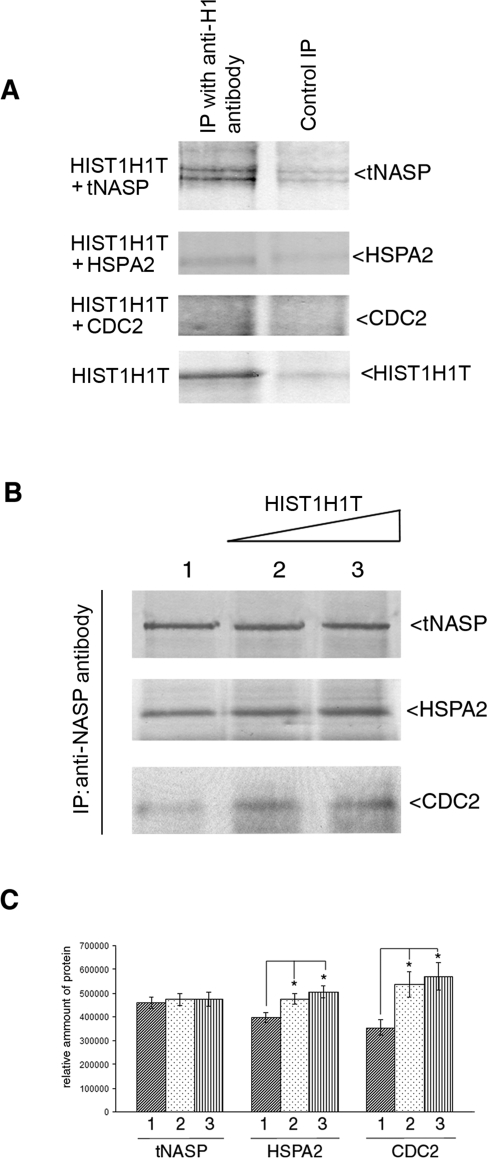
FIG. 5.
HIST1H1T affects the equilibrium of tNASP-HSPA2-CDC2 complex formation. A) Histone HIST1H1T binds to tNASP but not HSPA2 or CDC2 in vitro. Recombinant HIST1H1T was mixed with recombinant tNASP, HSPA2, CDC2, or HIST1H1T. All lanes were immunoprecipitated (IP) with anti-H1 antibody and stained with their respective antisera (IP with anti-H1). In negative controls (Control IP), HIST1H1T was not added except in the bottom panel (HIST1H1T), where HIST1H1T and a nonrelevant control antibody (anti-CYR61) were added. B) Increasing amounts of HIST1H1T were added to mouse testis lysates and tNASP immunoprecipitated. As HIST1H1T increased, HSPA2 and CDC2 increased: Lane 1: no HIST1H1T was added; lanes 2 and 3: 3 and 6 μM of recombinant HIST1H1T were added, respectively. C) Analysis of bands from B demonstrating significant changes (*) in the quantity of immunoprecipitated HSPA2 and CDC2 in the presence of increasing HIST1H1T. HSPA2: lane 1 vs. lane 2 (P < 0.02), lane 1 vs. lane 3 (P < 0.006). CDC2: lane 1 vs. lane 2 (P < 0.02), lane 1 vs. lane 3 (P < 0.02). The y-axis represents arbitrary units. Data are represented as mean ± SD.