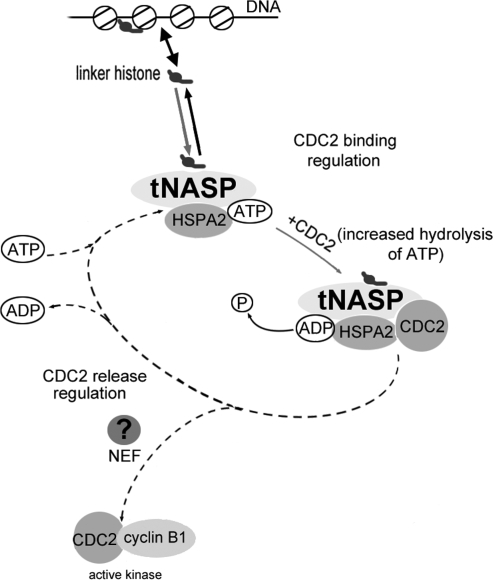
FIG. 7.
Regulation of HSPA2 chaperone cycle dynamics by linker histone-tNASP (modified from Bukau et al. [37] with permission from Elsevier). The cycle regulation starts with the association of linker histones with tNASP and the formation of a linker histone-tNASP-HSPA2 complex. The binding of CDC2 and increased ATP hydrolysis by HSPA2 causes the “locking in” of the substrate (CDC2) and formation of a linker histone-tNASP-HSPA2-CDC2 complex. The predicted release of CDC2 and ADP from the complex would mediate formation of active CDC2/cyclin B1 kinase (dotted line) and depend on nucleotide exchange factors, which are still to be identified.