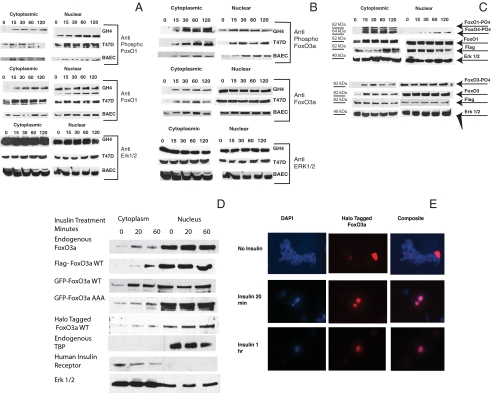
Figure 2.
Phosphorylation and compartmentalization of endogenous and transfected FoxO1 and FoxO3a: A and B, Endogenous FoxOs. GH4, T47D and BAE cells were plated in growth medium and switched to medium containing 10% charcoal-treated serum 24 h before the start of the experiment. Insulin, 1 μg/ml, was added for the indicated times, and the cells were rapidly washed with ice-cold saline and frozen at −75 C (<30 sec between 37 C and dry ice). Cytoplasmic and nuclear extracts were prepared as described in Materials and Methods, and the proteins were resolved by SDS-PAGE and transferred to nitrocellulose. A, The membranes were blocked and analyzed using antibodies to phospho-FoxO1 (Ser256 and 319) (top) and total FoxO1 (middle). The blots were also analyzed using antibody to ERK 1 and 2 as a loading control (bottom). B, The membranes were blocked and analyzed using antibodies to phospho-FoxO3a (Ser318/321) (top) and total FoxO3a (middle). The blots were also analyzed using antibody to ERK 1 and 2 as a loading control (bottom). C–E, Transfected FoxO1 and FoxO3a. C, GH4 cells were electroporated with 10 μg of Flag-FoxO1 (top) or 10 μg of Flag-FoxO3a (bottom). They were plated in growth medium and allowed to attach. The cells were switched to medium containing 10% charcoal-treated serum for 24 h. Insulin, 1 μg/ml, was then added for the indicated times. Cytoplasmic and nuclear extracts were prepared as described in Materials and Methods, and the proteins were resolved by SDS-PAGE and transferred to nitrocellulose. The membranes were blocked and analyzed using antibodies to phospho-FoxO1/O4 (Ser319/262) and total FoxO1 (top) or phospho-FoxO3a (Ser319/321) and total FoxO3a (bottom). The blots were also analyzed using antibody to Flag to show expression of exogenously expressed protein and with antibody to ERK 1 and 2 as a loading control. D, T47D cells were transfected with flag-FoxO3a wt, with GFP FoxO3a wt, GFP-FoxO3a AAA mutant, or HT-FoxO3a wt or untransfected for analysis of endogenous FoxO3a localization. After 24 h in medium containing charcoal-treated serum, the cultures were incubated with insulin for 20 min or 1 h or left untreated as controls. Cytoplasmic and nuclear extracts were prepared and analyzed by SDS-PAGE and blotted to nitrocellulose membrane. Anti-FoxO3a (Upstate), Anti-Flag (Sigma), and anti-GFP (Molecular Probes) were then used to show the distribution of FoxO3a. Extracts from HT-FoxO3a transfected cells were incubated for 30 min with HaloTMR ligand (Promega) that has a fluorescent label and covalently links to the HT-FoxO3a. Blots were stripped and blotted against ERK 1 and 2 as a loading control, TBP as an indicator of the specificity of the nuclear extract, or human insulin receptor as an indicator of the specificity of the cytoplasmic extract. E, T47D cells, grown on coverslips, were transfected using Lipofectamine 2000 (Invitrogen) with HT-FoxO3a wt and incubated with insulin for 20 min or 1 h or left untreated as a control. 4′,6-Diamidino-2-phenylindole fluorescence is in the left column, HT TMR ligand fluorescence is shown in the central column whereas the composite image is shown in the right column. Control cells are shown in the top row, whereas insulin treatment of 20 min is in the middle row and 1 h is in the bottom row. DAPI, 4′,6-Diamidino-2-phenylindole; WT, wild type.