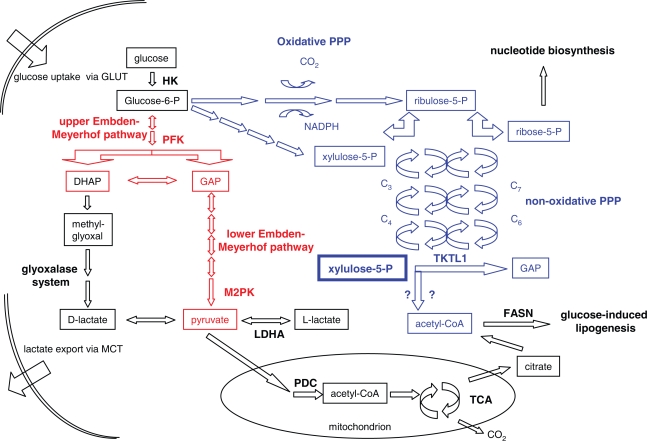
Figure 1.
Schematic illustration of different pathways of glucose metabolism in cancer cells. Glucose enters the cell via glucose transporter proteins (GLUT). Metabolic flux rates in different pathways are determined by the regulation of rate-limiting enzymes as described in the text, as well as by the balanced equilibrium of respective intermediates. Enzymes, reactions, and intermediates of the Embden-Meyerhof pathway are coloured in red, whereas those of the PPP are coloured in blue. Selected enzymes which are referred to in the text are listed at their respective positions. The oxidative branch of the PPP delivers ribose-5-phosphate for the biosynthesis of nucleotides, as well as NADPH for reductive biosynthesis, for the regulation of the redox state within the cell, and for the detoxification of ROS. Within the illustration of the non-oxidative PPP, C3, C7, C6, and C4 indicate sugar phosphate intermediates, which are interconverted in equilibrium reactions by the transketolase and transaldolase enzymes. Xylulose-5-phosphate, the concentration of which determines the flux rates through both major pathways, may be generated from glucose-6-phosphate via the oxidative or the non-oxidative branch of the PPP. It may serve as a substrate for TKTL1 in a putative cleavage reaction, which generates GAP and a 2-carbon unit, probably acetyl-CoA. Cytosolic acetyl-CoA is utilized for glucose-induced lipogenesis. GAP, which is also generated in the Embden-Meyerhof pathway, may be further metabolized either in the Embden-Meyerhof pathway, or via methylglyoxal to D-lactate. In cancer cells, the generation of L-lactate from pyruvate is predominantly catalyzed by LDHA. Both lactate stereo-isomers are in an equilibrium with pyruvate. Since the oxidation of pyruvate is frequently inhibited in cancer cells (see text), high amounts of lactate are exported via monocarboxylate transporters (MCT).