Figure 1.
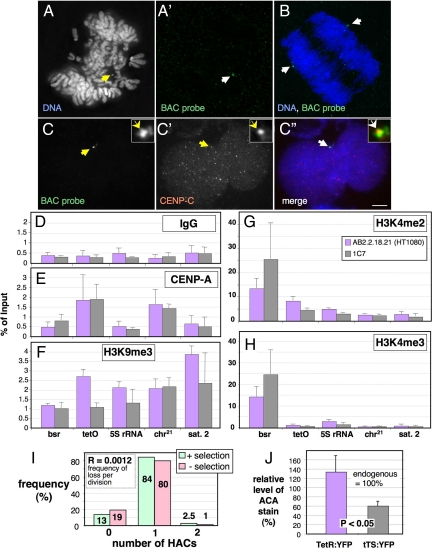
Isolation and characterization of a Hela-HT1080 hybrid cell line carrying a stable alphoidtetO HAC. (A) FISH with a BAC probe (green in A′) on mitotic chromosomes from the 1C7 cell line with 1 alphoidtetO HAC per nucleus (arrow in A). (B) FISH on anaphase 1C7 cell. The HAC (green, arrows in B) segregates with the endogenous chromosomes. Blue, DAPI staining for DNA. (C) Immuno-FISH with a BAC probe (C, green in the merged image in C″) and antibody against CENP-C (C', red in the merged image in C″) on an interphase 1C7 cell. CENP-C colocalizes with the BAC-probe on the HAC (arrows). Scale bar, 5 μm. (D–H) ChIP with control IgG antibody (D) and antibodies specific for CENP-A (E), trimethylated Lysine 9 on histone H3 (F), dimethylated Lysine 4 on histone H3 (G) and trimethylated Lysine 4 on histone H3 (H). Real-time PCR on the purified DNA was performed with primers specific for sequences in the actively transcribed Bsr (blasticidin resistance) gene on the BAC vector and the alphoidtetO array (tetO) forming the HAC centromere. As controls, primers specific for the DNA coding for rRNA (5S rDNA), endogenous chromosome 21 centromeric alphoid DNA (chr.21) and endogenous satellite 2 DNA (Sat 2), which is located in the pericentromeric heterochromatin of chromosomes 1 and 16, were used. Error bars, SEM of three independent ChIP experiments. (I) Analysis of HAC mitotic stability in 1C7 cells grown for 40 generations in the absence of blasticidin selection. The number of HACs per interphase nucleus was determined by FISH with a BAC probe. (J) Quantification of the amount of ACA human autoantibody (Earnshaw and Rothfield, 1985) staining associated with the alphoidtetO HAC in 1C7 cells fixed 48 h after transfection with TetR:EYFP and tTS:EYFP. Values are normalized to the average ACA staining of endogenous centromeres (100%). Error bars, SD.