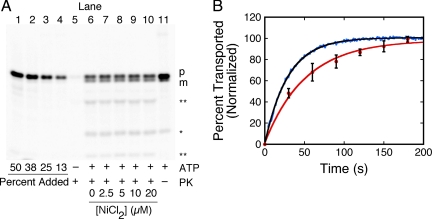
Figure 3.
Comparison of fluorescence- and gel-based transport assays. (A) Gel-based import assay. The proOmpA-HisC-Atto565 (50 nM) translocation yield, as measured with a protease (proteinase K = PK) protection assay analyzed by SDS-PAGE, was unaffected by NiCl2. Quantification standards (lanes 1–4) contain 13–50% of the precursor added to the import reactions (lanes 5–11). Transport reactions were quenched 5 min after IMV addition. Precursor (p) and mature (m) length protein bands are identified. The starred (*) band of lower MW is a digestion product that was included in the quantification of transport because: 1) it was not present in the precursor stock solution (lanes 1–4); 2) its appearance was ATP-dependent (cf. lane 5 with lane 6); 3) it was protease protected (lanes 6–10) and was generated in the absence of protease addition (lane 11); and 4) it was observed for the unpurified precursor protein (not shown), consistent with formation by an in vivo process. However, the double starred bands (**) were not observed in the absence of protease, suggesting that they were produced by the protease treatment. Bands were identified by Atto565 fluorescence emission. [IMV] (A280) = 1; [succinate] = 5 mM; [ATP] = 1 mM. (B) Comparison of the kinetics obtained from fluorescence- and gel-based transport assays. Shown are typical results obtained as in Figure 2D (blue, fluorescence assay) and Figure 3A (red, gel assay). Single exponential fits to the averaged data from three different IMV preparations yielded τ = 29 ± 4 s and τ = 46 ± 12 s, respectively. The proOmpA-HisC-Atto565 concentration (50 nM) was higher here than for most fluorescence-based assays to directly compare the fluorescence-based assay with translocation rates obtained from the lower sensitivity gel-based assay. Both transport assays were initiated by ATP addition and included 5 μM NiCl2.