Figure 4. Transient Absence of Ubx Nascent Transcripts in Recently Divided Cells.
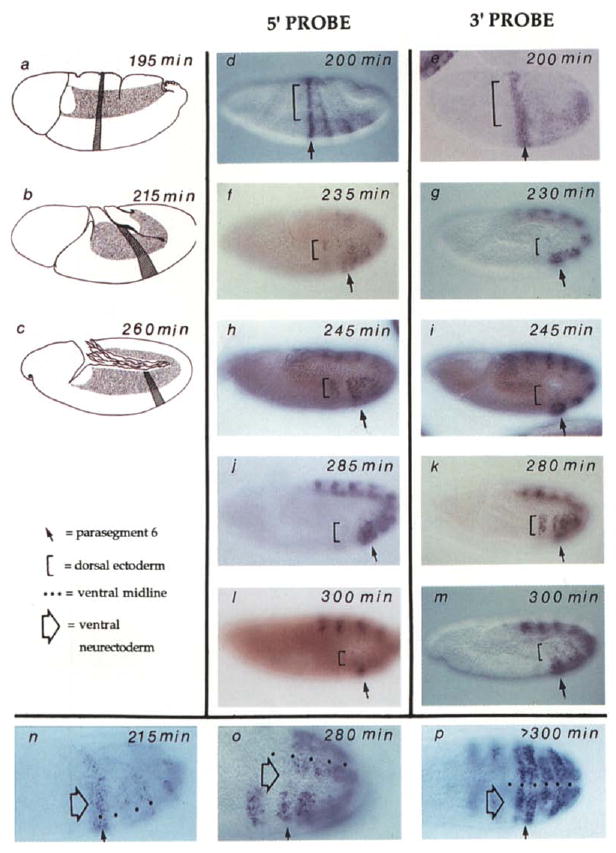
Wild-type embryos at various stages of cell cycle 14 and 15 are shown. In all panels except (n) to (p), anterior is left and ventral is down. The locations of PS6 (hatch stripe) and mitotic domain 11 (stippled region) are shown in the drawings on the left (a–c). These illustrate the shape changes during the movements of germband extension in which cells move ventrally to form the germband, which extends posteriorly, turns, and folds back upon itself. In the photographs, PS6 is indicated by an arrow and the dorsal ectoderm by a bracket (d–m) and a neurectoderm region just lateral to the ventral midline by a large open arrow (n–p). The age of the embryos in min AED was estimated by morphological criteria and progression of cell division domains (Campos-Ortega and Hartenstein, 1985; Foe, 1989) and is indicated on each panel. The embryos that are viewed from the side (d–m) are arranged according to age (top to bottom) so that staining with the 5′ probe (central column) can be compared to embryos at a similar stage stained with the 3′ probe (right column). In (d) and (e) are shown the patterns of Ubx expression as visualized about 10 to 15 min before division 14 begins in the dorsal ectoderm. Note that expression posterior to PS6 initiates later than PS6 expression (compare [d] to Figure 3) and is not yet evident with the 3′ probe in (e). Also, the embryo in (e) was flattened during slide preparation more than the other embryos in this display. In (f) and (g) are shown embryos shortly after completion of mitosis 14 in the dorsal ectoderm. No 3′ signal is seen (g) in this domain, while some heterogeneity (staining and nonstaining cells) is seen in this region with the 5′ probe. As discussed in the text, disappearance of signal is associated with progress through mitosis, and the heterogeneity of expression with the 5′ probe (f) is due to reappearance of signal in cells that have progressed further into cycle 15. Note that the brownish red color of the embryos in (f) and (I) appeared during observation under UV light and is not relevant to the hybridization signal. In (h) and (i) are shown embryos 20 min after mitosis 14 in the dorsal ectoderm. A strong signal is seen in this region with the 5′ probe but not with the 3′ probe. In (j) and (k) are shown embryos about 50 min after mitosis in the dorsal ectoderm. A strong signal is in the dorsal ectoderm with both 5′ and 3′ probes. Mitosis 15 has begun in some regions of the dorsal ectoderm of the embryo shown in (j) (fluorescence of Hoechst 33258–stained DNA allowed visualization of these mitoses; data not shown) and signal with the 5′ probe has been lost in some regions, notably in PSS. In (I) and (m) are shown embryos in which most of the cells of the dorsal ectoderm have progressed through mitosis 15 and signal is again absent from this region with both 5′ and 3′ probes. Note that the faint mottled expression in the dorsal ectoderm of the embryo in (m) is due to staining of cells in a focal plane below the ectoderm. Such nonectodermal cells divide according to a different schedule. In (n), (o), and (p) are shown embryos oriented so that the ventral region is visible. The dots mark the ventral midline. These embryos have been hybridized with the 3′ probe. At stages prior to mitosis 14 (n) and well after mitosis (p) the signal spans the ventral region of the embryo. In (o) there is an interruption in the signal in a region just lateral to the midline (large open arrow). This corresponds to mitotic domain N where mitosis has recently been completed. The signal is mottled in the more ventral region Oust adjacent to the midline marked by dots), which corresponds to mitotic domain M where division is in progress (some cells have divided and others have not).
