Abstract
This review focuses on saccade research with adult psychiatric patients. It begins with an introduction of the various types of saccades and the tasks used to evoke them. The functional significance of the different types of eye movements is briefly discussed. Research findings regarding the saccadic performance of different adult psychiatric patient populations are discussed in detail, with particular emphasis on findings regarding error rates, response latencies, and any specific task parameters that might affect those variables. Findings regarding the symptom, neurocognitive, and neural correlates of saccadic performance and the functional significance of patients’ saccadic deficits are also discussed. We also discuss the saccadic deficits displayed by various patient groups in terms of circuitry (e.g. cortical/basal ganglia circuits) that may be implicated in the underlying pathophysiology of several of these disorders. Future directions for research in this growing area are offered.
The saccadic eye movement system is responsible for rapid eye movements that bring the image of an object onto the fovea (see glossary) (Leigh & Zee, 1983). Saccades are conjugate, ballistic eye movements that are characterized by short reaction times, typically around 200 msec., and brief durations, normally between approximately 20 and 120 msec. (Leigh & Zee, 1983; Gouras, 1985; Engelken, Stevens, & Enderle, 1989). In humans, saccades can reach speeds of up to 600 to 700 deg/sec., whereas monkeys can produce saccades that are nearly twice as fast (Gouras, 1985; DeRenzi, 1988). Normal saccadic eye movements are characterized by an invariant relationship between their peak velocity and their size; the amplitude-peak velocity relationship is referred to as the main sequence (Bahill & Stark, 1975).
Accuracy of saccades depends on the location of a target on the retina as well as the position of the eyes in the orbit (Gouras, 1985). There are two major classes of saccades, namely, those that are externally triggered and automatic, and those that are internally initiated and volitional (Lasker, Zee, Hain, Folstein, & Singer, 1987). Although saccadic eye movements are typically elicited by the sudden appearance of a novel auditory or visual stimulus that attracts the subject’s attention (reflexive saccades), they can be produced on command, in the dark, to remembered targets, during scanning or searching of stationary visual scenes, or with closed eyes (Leigh & Zee, 1983; Gouras, 1985; DeRenzi, 1988; Wirtschafter & Weingarden, 1988). Saccades can be described in terms of their reaction time, namely, the time elapsing from the command signal to shift one’s gaze until the beginning of the saccade. Saccades are also described in terms of their velocity and accuracy.
Diefendorf and Dodge (1908) were the first investigators to report on the functioning of the saccadic system in schizophrenia patients. Since then, interest in the study of saccadic eye movements in schizophrenia has risen steadily and now includes a focus on volitional saccades as well as reflexive ones. Saccadic eye movements can be measured reliably and precisely. As a result of basic research including single unit recordings and lesion studies as well as clinical research and functional imaging, there is a considerable body of knowledge regarding the neurophysiology of the saccadic eye movement subsystem. Thus, the study of saccadic eye movements in psychiatric patient groups can provide a “window into the brain” of affected individuals. Over the past three decades, there has also been an increase in the number of studies of saccadic performance in other psychiatric patient groups. Much of the impetus for the focus on saccadic eye movements in these populations comes from the fact that saccades provide a noninvasive yet accessible means of investigating psychomotor functioning as well as higher order cognitive processes and their underlying neural mechanisms.
In the following review, we summarize the extant literature regarding the study of saccadic eye movements in adult psychiatric patients. In order to appreciate the growing complexity of the area and the various ways in which psychiatric patient groups differ in terms of their saccadic task performance, we introduce the major saccadic paradigms prior to summarizing the studies for each diagnostic group. Given the disproportionately large corpus of literature on saccadic performance in schizophrenia and schizophrenia spectrum populations, this area will receive particular emphasis in our review. Findings regarding the symptom, neurocognitive, and neural correlates of saccadic performance, as well as hypotheses regarding the functional significance of patients’ saccadic deficits are also discussed. In the final section of the review, we integrate across findings, and provide a comparative analysis of saccadic deficits in the different psychiatric disorders. This discussion is used as an opportunity for hypothesis generation and recommendations for future directions.
Different Types of Saccade Paradigms
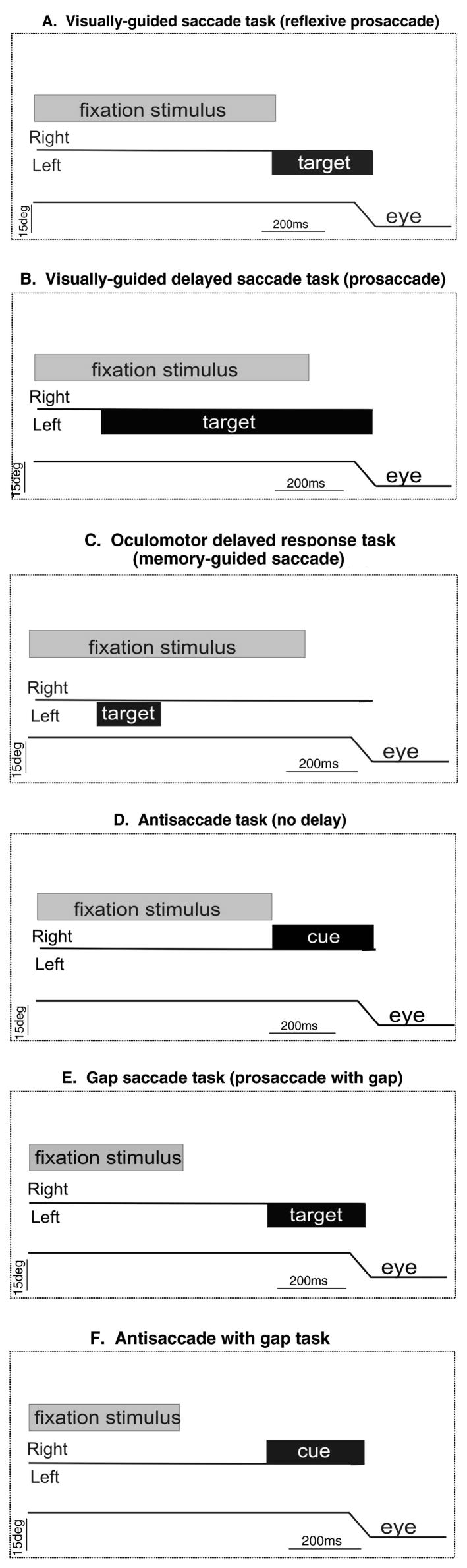
Because of the versatility of saccades, a number of different behavioral tasks have been developed over the years to probe different underlying mechanisms. Below we review some of the most commonly used saccadic eye movement tasks. The different saccade tasks include visually-guided, memory-guided, gap, overlap, and antisaccade tasks. Each task has different demands allowing for the assessment of the integrity of brain pathways in health and disease. For example, to assess the integrity of saccade-generating circuitry subjects may perform a visually-guided saccade task. In this task (Figure 1A), a fixation stimulus appears initially at the center of a screen. After a timed fixation period a second stimulus (target) appears at a peripheral location. At the same time, the fixation stimulus disappears and the subject must initiate a saccade to the peripheral target. In this most simple version of the visually-guided saccade task, the appearance of the peripheral target and the removal of the central fixation occur simultaneously and as such, the task is also referred to as a reflexive visually-guided saccade or prosaccade. In one version of the prosaccade task, known as the overlap prosaccade task, the cue and the central fixation stimulus appear together, i.e., overlap. Imposing a delay between the time of the appearance of the target stimulus and the disappearance of the fixation stimulus cueing the saccade is referred to as a delayed visually-guided saccade (Figure 1B).
Figure 1.
Saccadic eye movement tasks. Each panel shows the salient events occurring in different saccade tasks over time. The line marked Right and Left in each panel indicates the hemifield in which the target appears. The bottom line labeled ‘eye’ in each panel is a schematic of the eye position. Upward deflections of the eye position trace indicate rightward eye movements and downward deflections indicate leftward eye movements. The grey bar in each panel labeled ‘fixation’ indicates the onset and duration of the fixation stimulus, usually located in the center of a screen. The black bar labeled ‘target’ in each panel indicates the onset and duration of the target which is usually located in the periphery.
A. Visually-guided saccade task. The fixation stimulus appears (grey bar) and the subject is required to maintain gaze on its location. After a fixation time a peripherally located target (black bar) appears and at the same time the fixation stimulus disappears. The subject is required to make a saccade to the target. This task is also referred to as a reflexive prosaccade.
B. Visually-guided, delayed-saccade task. The fixation stimulus appears and after a fixation time the target appears. A further delay is imposed during which the subject maintains fixation. After the delay, the fixation stimulus disappears signaling the subject to make a saccade to the target.
C. Memory-guided saccade task. The fixation stimulus appears and the subject is required to maintain gaze on its location. After a fixation time a target appears transiently. The subject is required to maintain fixation and only after another delay does the fixation stimulus disappear signaling the subject to make a saccade to the location of the previously flashed target. This task is also referred to as the oculomotor delayed response task (ODR).
D. Antisaccade task. This task is identical to the reflexive saccade task except that a peripheral stimulus appears in one hemifield (now called cue) and the required saccade is to the opposite hemifield. In this example, the cue appears in the right hemifield and the saccade is leftward.
E. Gap saccade task. The fixation stimulus appears and after a fixation time it disappears. The subject maintains gaze at the center where the fixation stimulus was. After a ‘gap time’ a second stimulus appears in the periphery and the subject is required to make a saccade to the location of the second stimulus. Note that this task differs from all others in that the cue to make a saccade is the onset of the peripheral stimulus whereas in the other tasks the cue to make a saccade is the offset of the fixation stimulus.
F. Antisaccade with gap task. This task is a combination of those shown in D and E. The peripheral stimulus (cue) appears in one hemifield and the saccade occurs toward the opposite hemifield. The onset of the cue occurs after a ‘gap time’ in which the fixation stimulus is no longer present on the screen.
A commonly used type of delayed-saccade task is referred to as the oculomotor delayed-response (ODR) or memory-guided saccade task (Figure 1C). For this subjects fixate a centrally-illuminated stimulus. While the fixation stimulus is illuminated, the appearance of a second peripheral stimulus occurs transiently. Following a delay-period of up to 10 seconds, the central fixation stimulus disappears signaling the subject to make a saccade to the remembered location of the previously flashed stimulus. These saccades are often referred to as memory-guided to distinguish them from visually-guided ones (Hikosaka and Wurtz 1983). The ODR task provides a test of spatial working memory (see glossary) as well as the ability to suppress a reflexive saccade.
In addition to prosaccade tasks, sometimes also referred to as refixation saccade tasks, there are tasks that dissociate the location of the cue from the location of the required saccadic eye movement. The antisaccade task (see glossary) is the most notable example. In the standard version of the antisaccade task (Hallet, 1978; Hallet & Adams, 1980), the participant starts by fixating on a centrally-illuminated stimulus. After a fixation interval, the stimulus disappears and at the same time a stimulus appears at a peripheral location. The task of the subject is to make a saccade to the location opposite the appearance of the stimulus. Thus, to perform the task correctly the subject must inhibit a prepotent (overlearned and automatic) response, i.e., a reflexive saccade, and generate a volitional saccade in the opposite direction. The saccade made in the opposite direction is called the antisaccade (Figure 1D).
There are several variants of the antisaccade task. Some include a warning sound prior to the cue appearance and some vary the timing between the offset of the central stimulus and the onset of the peripheral cue. One common variation of the antisaccade task is the gap version. In this version the central fixation stimulus disappears shortly before the peripheral target appears (Figure 1F). The temporal gap between the offset of the central stimulus and the onset of the peripheral stimulus may place greater demands on the mechanisms of voluntary inhibition (see glossary) than the standard version of the antisaccade task. A second version of the antisaccade task increases the time in which the cue and the central fixation stimulus appearance occur together or overlap (Sereno & Holzman, 1993). The overlap condition is an easier version of the antisaccade task. Across all of the variants of the antisaccade task paradigm, three processes are necessary for the successful execution of the task: first, there must be a covert shift of exogenous attention; second, the subject must inhibit the reflexive saccade to the cue; and third, the subject must transform the spatial position of the cue to the spatial position of the saccade goal and then initiate a voluntary saccade in the opposite direction to the target movement (Hallet, 1987; Harris et al, 2006).
The gap saccade paradigm is unique among the saccadic tasks in that the cue to make the saccade is the appearance of the target. In contrast, most other saccade tasks are cued by the removal of the fixation stimulus (Figure 1E). In the gap paradigm, the centrally located stimulus appears and after a delay, disappears. During this time the subject maintains gaze at the center of the screen despite the absence of the central fixation. The appearance of the peripheral target is the cue for the subject to initiate a saccade to the target location (Saslow, 1967). The gap paradigm is used to elicit express saccades, visually-guided saccades with latencies between 80 and 130 msec (Fischer & Ramsperger, 1984). The reduction in saccade latency in this task (compared to the condition in which the fixation stimulus and the target stimulus appear with some temporal overlap) is known as the gap effect (Saslow, 1967; Reuter-Lorenz, Hughes, & Fendrich, 1991; Fisher & Weber, 1993).
In predictive saccade tracking tasks, subjects are required to keep their eyes on a target that moves regularly back and forth between two known locations, i.e., left and right of center, remaining in each position for a period of time (e.g. 500 msec). Predictive saccade tracking tasks are used to assess participants’ ability to adjust their oculomotor response to a predictably moving visual stimulus. Typically, within a few target presentations, healthy subjects are able to generate anticipatory saccades that are characterized by reduced latencies (Bronstein & Kennard, 1987; Spengler et al., 2006). In contrast to prosaccade paradigms, which assess reflexive eye movements, the antisaccade, ODR, and predictive paradigms assess intentional saccadic movements. Visually guided prosaccade tasks provide an external representation of the goal. In contrast, performance of other tasks (i.e., antisaccade, ODR, and predictive) depends upon an internal representation of the target position. Readers are referred to the Hutton (2008) paper, in this volume for a detailed discussion of the cognitive control of saccadic eye movements.
Intentional or volitional saccadic eye movements can also be examined through the use of scanpaths or visual search tasks. Repetitive sequences of saccades or scanpaths are generated in order to examine subfeatures of a visual scene (Yarbus, 1967; Stark, 1983). In visual search tasks, subjects are required to move their gaze as they view a complex visual scene or compare visual stimuli. These tasks require focused visuospatial attention as well as the ability to use a task goal to guide subsequent behavior, i.e., working memory (Kennard, 2002).
This review will focus on findings from saccade research with adult psychiatric patients. Extant literature pertaining to saccadic performance in schizophrenia and schizophrenia-spectrum disorders will be discussed first, followed by research on patients with mood disorders. Data from studies that combined samples of mood disordered patients will be considered prior to reviewing studies based on select samples of patients with major depressive disorder, bipolar disorder, and borderline personality disorder. Findings from studies of individuals with attention deficit hyperactivity disorder (ADHD), Tourette’s syndrome, and obsessive compulsive disorder (OCD) are also summarized. In this review, research findings are organized according to the disorder discussed, rather than the type of eye movement, because typically investigators administered more than one oculomotor task in order to assess the functioning of the participants’ saccadic system. By manipulating the demands of the tasks requiring saccade generation, one can assay cognitive processes such as goal setting, response generation, response inhibition, prediction, and spatial working memory. Indeed, through comparisons of performance patterns across tasks, investigators have been able to draw conclusions and propose models about the circuitry that may be implicated in the underlying pathophysiology of several of these disorders.
Saccadic Performance in Schizophrenia and Schizophrenia Spectrum Disorders
Most of the studies of saccadic performance in psychiatric samples have focused on schizophrenia patients. Additionally, several studies have investigated performance on saccadic tasks in schizophrenia spectrum populations such as schizotypal personality disorder and/or individuals at heightened risk for the later development of schizophrenia and schizophrenia spectrum disorders. In this review, studies focusing on schizophrenic patient samples will be addressed first prior to discussing the findings involving individuals with schizophrenia spectrum disorders.
1. Saccadic Refixation in Schizophrenia Patients
Saccadic performance is typically described in terms of static characteristics (e.g., accuracy, corrective movements), saccadic reaction time, and dynamic characteristics such as velocity, duration, and gain (Becker, 1989; Mahlberg et al., 2001). Overall, schizophrenia patients display normal saccadic eye movements to peripherally presented visual cues that follow the offset of a central fixation point (Barton et al, 2002; Clementz et al., 1994; Diefendorf & Dodge, 1908; Fukushima et al., 1990; Gooding, Iacono, & Grove, 1997; Gooding, Mohapatra, & Shea, 2004; Hutton et al., 1998; Iacono, Tuason, & Johnson, 1981; Klein et al., 2000; Hutton et al., 2002; Levin et al., 1982; Mahlberg et al., 2001; Tendolkar et al., 2005; but see Boudet et al 2005, Crawford et al., 1995; Grootens et al., 2008). It is noteworthy that in the Crawford et al. (1995) study, the schizophrenia patients were unmedicated, which may have accounted for less accurate responses. Nieuwenhuis, Broerse, Nielen, and deJong (2004) observed that schizophrenia patients displayed normal performance on the simple version of the prosaccade task, in which saccades were explicitly required. However, when administered a more difficult version of a prosaccade task, i.e., a cued target discrimination task in which subjects could improve their discrimination performance by making an eye movement (prosaccade) towards a cue, schizophrenia patients performed significantly worse than controls. Considered together, these findings suggest that patients’ basic saccadic generation circuitry is intact, but a higher order process is impaired.
The findings regarding schizophrenia patients’ ability to locate displaced targets accurately are equivocal. Although there are some reports ( Fukushima et al., 1988; Iacono et al, 1981; Levin et al., 1982; Gooding et al., 1997) that this ability is intact in schizophrenia, several other studies (Cegalis et al., 1982; Clementz et al., 1994; Levin et al. 1981; Mahlberg et al., 2001; Mather & Putchat, 1983; Moser et al., 1990; Ross et al., 1988; Schmid-Burgk, Becker, Diekmann, Jurgens, & Kornhuber, 1982) indicate that schizophrenia patients display saccadic dysmetria. In some samples (Clementz et al., 1994; Levin et al., 1981; Moser et al., 1990), the hypometric (see glossary) saccades were limited to rightward visually guided saccades. Differences in terms of eye movement recording techniques, as well as differences in target stimuli displacement, predictability, and timing have been posited to account for the apparent discrepancy in findings.
Schizophrenia patients’ predictive saccades have been reportedly less accurate than controls in some studies (e.g., Crawford et al. 1995; Hommer, Clem, Litman, & Pickar, 1991; Krebs et al., 2001) but not in others (McDowell, Clementz, & Wixted, 1996). In their assessment of unmedicated schizophrenia patients’ performance on a predictive saccade task, Krebs’ group (2001) evaluated the subjects’ non-anticipated saccades, which are externally elicited, separately from their anticipated saccades, which are internally elicited. The patients did not differ from controls in terms of their non-anticipated saccades’ latency, peak velocity, or gain, nor did the groups differ in terms of their final eye position. Krebs et al. (2001) interpreted the observation that schizophrenia patients’ anticipated saccades were characterized by hypometria as indicative of an impairment in internally based motor planning. In contrast, Sailer et al. (2007) observed that acutely ill schizophrenia inpatients did not differ from healthy controls in terms of the accuracy (gain) of their correct and incorrect anticipatory saccades. However, they noted that the patients generated significantly more correct anticipatory saccades than controls; the investigators viewed this enhanced predictive saccade activity as indicative of an impaired gating mechanism for predictive saccades. A more recent investigation of medicated and unmedicated patients revealed that schizophrenia patients’ anticipated predictive saccades were characterized by lower primary and final gain, and decreased maximum velocity relative to controls (Amado et al., 2008). Moreover, the schizophrenia patients’ nonanticipated saccades were characterized by lower primary and final gain. The schizophrenia patients did not differ from healthy matched controls in terms of the latencies for either the anticipated or nonanticipated saccades (Amado et al., 2008).
On simple prosaccade tasks and predictive tracking tasks, the saccadic reaction times of schizophrenia patients are typically normal ( Fukushima et al., 1988; Levin et al., 1982; Mather & Putchat, 1982; Tendolkar et al., 2005; Thampi et al., 2003; Grootens et al., 2008). Schizophrenia patients may display significantly prolonged saccadic response latencies under conditions of considerable task complexity (Done & Frith, 1984; Mackert & Flechtner, 1989; Schmidt-Burghk et al, 1982). For example, in a complicated choice paradigm in which subjects were required to move their eyes either left or right as directed by an imperative signal and report upon the characteristics of a peripheral stimulus, Done and Frith (1984) observed that schizophrenia patients had significantly longer saccadic latencies. The Mackert and Flechtner (1989) task was similarly complex; fast-changing saccadic stimuli were presented in varying positions for varying durations (800 to 1200 msec.) randomly chosen and led to prolonged saccade latencies.
In general, investigations of average and peak saccadic velocities (Levin et al, 1981; Levin et al, 1982; Mather & Putchat, 1983; Yee et al, 1987; Ross et al, 1988) indicate that schizophrenic patients’ velocities are within normal limits, though there has been one report (Cegalis, Sweeney & Dellis, 1982) suggesting that they are slower. The differential findings of Cegalis et al (1982) may be due to their use of a more difficult refixation task, in which the signal alternately disappeared and reappeared on opposite sides of the screen. Among more recent reports (Mahlberg et al, 2001; Ramchandran et al, 2004; Thampi et al, 2003), the findings are mixed. While Thampi et al (2003) who included both treatment responsive and treatment resistant schizophrenia patients, found no differences between schizophrenia patients and controls in terms of peak saccadic velocity on the prosaccade task, the other two groups (Mahlberg et al., 2001; Ramchandran et al. 2004) found that schizophrenia patients display aberrant peak velocities during saccadic refixation tasks. It is noteworthy that saccadic peak velocity is affected by sedative medications (Van Opstal & Van Gisbergen, 1987); readers are referred to Reilly et al. (2008) for discussion of pharmacological treatment effects on eye movement control. Mahlberg et al (2001) observed that the saccadic eye movements of the 38 unmedicated schizophrenic subjects were characterized by significantly increased peak velocity on both the “prosaccade task” and the predictive saccadic task.
Ramchandran et al (2004) noted that the medicated schizophrenia patients’peak velocities could be normal or aberrant, depending on their latency, with the velocity declining with increasing latency. At latencies greater than 300 msec, the peak velocities were significantly reduced relative to the healthy controls. The investigators attributed the patients’ saccadic abnormality to a failure of the continued target presence to sustain saccade-related neural activity. While these data could be related to a larger body of literature regarding perceptual dysfunction in schizophrenia, particularly the premature decay of sustained pattern information (Ramchandran et al, 2004), it also suggests that schizophrenia patients’ peak saccadic velocities are quite malleable and easily affected by task parameters.
2. Antisaccade Task Performance in Schizophrenia Patients
In addition to the basic measures of saccadic task performance, namely, percent of correct saccadic responses (and conversely, percent of directional errors), latency to initiate correct response, and saccadic gain, antisaccade task performance can also be described in terms of percent of corrected errors. Antisaccade errors reflect failures of response suppression and are considered the most reliable measure of antisaccade performance (Ettinger et al, 2003). The main antisaccade abnormality reported in schizophrenia is an elevated rate of reflexive saccade error rates.
Fukushima et al. (1988) were the first investigators to administer an antisaccade task paradigm to schizophrenia patients. The finding that schizophrenia patients display impaired antisaccade task performance relative to healthy controls is a robust one, with over 50 replications to date. Error rates in schizophrenia patients vary from approximately 20% to 75%, depending upon the task parameters. Aberrant antisaccade task performance has been observed in patients with recent onset and/or first-episode schizophrenia (Boerse, Crawford & den Boer, 2002; deWilde, Dingemans, Boeree & Linszen, 2008; Ettinger, Kumari, Chitnis et al., 2004; Grootens et al., 2008; Hutton et al., 2002; Hutton et al., 1998) as well as chronic schizophrenia (Boudet et al., 2005; Curtis, Calkins, & Iacono, 2001; Fukushima et al., 1988) and remitted schizophrenia (Curtis, Calkins, Grove, Feil, & Iacono, 2001). Increased proportions of antisaccade errors, operationally defined as glances made in the same direction as the target, have been observed in never-medicated schizophrenia patients (Harris et al., 2006) and unmedicated patients (Mahlberg, Steinacher, Mackert & Flechtner, 2001) as well as in patients receiving antipsychotic treatment.
Schizophrenia patients consistently produce fewer correct responses on the antisaccade tasks, regardless of the version of the paradigm (e.g. Allen et al., 1996; Clementz et al., 1994; Crawford et al., 1998; Curtis et al., 2001; Fukushima et al., 1988; Fukushima et al., 1990; Gooding et al., 1997; Gooding & Tallent, 2001; Hutton et al., 2002; Klein et al., 2000; McDowell et al., 1999; Reuter et al, 2005; Sereno & Holzman, 1995; Thaker et al., 1990). This robust finding was recently replicated in the largest investigation of schizophrenia patients to date, in which 141 schizophrenia patients and 193 community controls were compared (Radant et al., 2007). The overlap between the offset of the fixation and the antisaccade cue stimulus onset affects the magnitude of the difference between schizophrenia patients and nonpsychiatric controls; a better separation is achieved in the overlap condition than in the standard version of the antisaccade task (McDowell & Clementz, 1997), and a ‘far overlap’ version reportedly provides larger separations (i.e., 5–6 sigma) than ‘near overlap’ versions (McDowell, Myles-Worsley, Coon, Byerley, & Clementz, 1999). The increased effect size is likely due to the increase in task difficulty that occurs when the cue is spatially further from the fixation point.
The finding of increased proportions of directional errors in the antisaccade task is robust across different recording techniques, including electro-oculography (EOG; Grootens et al., 2008; Muller et al., 1999;), infrared recording (IR; Ettinger et al., 2004a,b; Gooding et al., 2004), and the magnetic induction technique (Barton et al., 2002; Nieman et al., 2007; Ramchandran et al., 2004). Although schizophrenic patient groups make a significantly higher percent of directional errors in antisaccade tasks, they usually do not differ from healthy controls in terms of their percent of corrected errors (Gooding & Tallent, 2001; Polli et al., 2008); this is important, because it indicates that they understand the task instructions and are sufficiently motivated to perform the task. Several models have been offered to account for the increased frequency of antisaccade errors displayed by schizophrenia patients; these accounts will be discussed and critiqued following review of the extant literature regarding the schizophrenic individuals’ saccadic abnormalities. This reflects our belief that the pattern of performance across tasks may provide greater insights regarding the functional significance of schizophrenia patients’ antisaccade task deficits vis a vis the mechanisms underlying the disorder.
Some studies also measure the spatial accuracy of the correctly performed antisaccade, by measuring the positions of the primary saccade and the final eye position and comparing them with the target position. Schizophrenia patients have a tendency to display reduced spatial accuracy, whereby the primary saccade and corrective saccade undershoot the target (Burke & Reveley, 2002). Several studies have reported that schizophrenia patients show reduced spatial accuracy of their correct antisaccades (Ettinger, Kumari, Chitnis et al., 2004; Ettinger, Kumari, Crawford et al., 2004; Karoumi et al., 1998; McDowell, Myles-Worsley, Coon, Byerley & Clementz, 1999; Ross et al., 1998). Reduced spatial accuracy of the antisaccade response may reflect an impaired ability to generate saccades and/or a perceptual deficit thereby limiting an accurate internal representation of the spatial layout.
Some versions of the antisaccade task paradigm do not require subjects to move their eyes as fast as possible, so investigators do not measure response latency. However, the latencies of correct antisaccade responses are thought to reflect the processing speed for planning and generating voluntary action (Harris et al, 2006). Given what is required to correctly perform the antisaccade task, the response latency may provide an index of the efficiency of visual processing, response suppression, and/or response generation. Investigators (Barton et al., 2002; Curtis et al., 2001; Crawford et al., 1998; deWilde et al., 2008; Fukushima et al., 1990; Karoumi et al., 1998; Klein et al., 2000; Muller et al., 1999; Sereno & Holzman, 1995; Spengler et al., 2006; Thaker et al., 1990) have reported that schizophrenia patients display increased saccadic latency to correct antisaccade task responses (but see Clementz, McDowell & Zisook, 1994; Hutton et al., 2002; Maruff et al., 1998). It is noteworthy that Curtis et al. (2001) observed that acutely ill schizophrenia patients, though not remitted patients, showed significantly increased latencies on correct antisaccade trials. Although Ettinger and colleagues (2004a) noted no difference between the first episode psychotic patients and healthy controls in terms of response latency, it is unclear how many of these patients met diagnostic criteria for schizophrenia.
Investigators have examined whether the saccadic latencies can be manipulated in schizophrenia patients as they are in healthy controls, i.e., whether individuals with schizophrenia will display the ‘gap effect’. The literature is consistent in indicating that schizophrenia patients show latency reductions on visually guided saccadic tasks in which there is a temporal delay after the fixation spot is extinguished and prior to the appearance of an eccentric target (Clementz, 1996; Reilly, Harris, Khine, Keshavan & Sweeney, 2008; Sereno & Holzman, 1993; Smyrnis et al., 2004). However, findings are mixed regarding whether the gap effect is present in the antisaccade task. While in some investigations schizophrenic individuals displayed reduced latency in the gap antisaccade condition similarly to healthy individuals (McDowell & Clementz, 1997; Reilly et al., 2008), in another study, half of the patients failed to show the gap effect (Smyrnis et al., 2004). Schizophrenia patients reportedly do not differ from nonpsychiatric controls in terms of their latencies to error responses (Boudet et al., 2005; Curtis et al., 2001; McDowell & Clementz, 1997), though this has been studied considerably less often. However, as Klein (2001) demonstrated using Principal Components Analysis, latencies to correct responses taps an aspect of performance that is distinct from latency to error responses, and therefore should be studied separately.
Schizophrenic patients’ aberrant antisaccade task performance cannot be attributed to state-related factors. Supportive evidence for the trait-like nature of this deficit is derived from investigations of medication effects on antisaccade performance, studies of clinical correlates of schizophrenia patients’ antisaccade task performance, and longitudinal studies of antisaccade task performance in schizophrenia patients. Pharmacological agents exert effects on antisaccadic performance in both healthy control subjects and schizophrenia patients. Antisaccade performance is sensitive to various psychopharmacological manipulations in healthy participants, including lorazepam (Green & King, 1998; Green, King, & Trimble, 2000), alcohol (Khan et al., 2003), nicotine (Rycroft et al., 2007), amphetamine (Dursun et al, 1999), and modafinil (Rycroft et al., 2007). Readers are referred to Reilly et al. (2008) for a detailed discussion of this topic.
Most studies examining the relationship between psychotropic medications and antisaccade task performance ( Fukushima et al., 1988; McDowell & Clementz, 1997; Broerse et al., 2002; Ettinger et al., 2003; Raemakers et al., 2002; Wonodi et al., 2004; Allen et al., 1996; Karoumi et al., 2001; Nkam et al., 2001; Maruff, Danckert, Pantelis & Currie, 1998) conclude that the task deficits are not attributable to medication exposure. In schizophrenia patients, risperidone has been observed to improve error rates in some schizophrenia patients (Burke & Reveley, 2002; Hutton, 2002; but see Harris et al., 2006). There have also been reports that antisaccade performance in schizophrenia may improve with nicotine (Depatie et al., 2002; Larrison-Faucher et al., 2004). However, to date, no pharmacological agent has been able to normalize schizophrenic individuals’ excessive number of directional errors on antisaccade compared to nonpsychiatric controls.
Despite the fact that the group difference in directional errors is a more robust observation, some investigators have examined the effects of pharmacologic agents on antisaccade latencies. In their comparison of medicated and unmedicated first-episode schizophrenia patients, Hutton et al (1998) observed increased error rates for both patient groups but prolonged response latencies only in the treatment-naïve group. Yet in another investigation of treatment-naïve patients (Muller et al., 1999) showed that treatment had no effect on either antisaccade accuracy or latency.
There have been relatively few longitudinal studies of antisaccade task performance in schizophrenia patients. Antisaccade task accuracy in schizophrenia patients shows high retest stability (i.e., r’s ranging from .75 to .90) for intervals up to one year (Thaker et al., 1990;). Chronic schizophrenia outpatients who were tested three years after their initial assessment displayed high test-retest reliability in terms of their antisaccade accuracy, despite changes in their clinical status and medication regimes (Gooding, Mohapatra, & Shea, 2004). Longitudinal findings based upon a sample of antipsychotic-naïve patients were consistent with those of Gooding and colleagues (2004). Harris et al. (2006) followed 41 healthy community-based controls and 39 antipsychotic-naïve patients who met DSM-IV criteria for either schizophrenia or schizoaffective disorder, depressive type over a one year period. Although the patients’ antisaccade performance gradually improved over the course of the year, they showed elevated error rates relative to the healthy controls across all time points. This finding indicates that the patients’ impaired ability to plan and initiate context-appropriate responses was stable over time.
Performance on Memory-Guided (Oculomotor Delayed Response) Tasks
Schizophrenia patients’ oculomotor delayed response task performance is characterized by excessive anticipatory saccades during the delay period (Camchong et al., 2006; Everling et al., 1996; Hommer et al., 1991; Landgraf et al., 2008; McDowell & Clementz, 1996; McDowell et al., 2001; Park, 1997; Park & Holzman, 1992). In memory-guided saccades, schizophrenia patients typically display increased latencies and/or decreased gain (Everling et al., 1996; Landgraf et al, 2008; McDowell & Clementz, 1996; McDowell & Clementz, 2001; Muller et al., 1999; Park & Holzman, 1992, 1993; Ross et al., 1998). Given that schizophrenia patients displayed impaired performance on oculomotor delayed response tasks yet intact performance on visually guided saccade tasks, Park and Holzman (1992, 1993) suggested that schizophrenia patients had a selective deficit in their representational processing.
Consideration of patients’ performance across tasks provides insight into the nature and extent of their oculomotor deficits. Although schizophrenia patients display impairments in terms of inhibiting a reflexive saccade during an antisaccade task, data from a study by Hutton et al. (2002) suggest that the schizophrenia patients’ saccadic inhibition deficit may not be global. That is, schizophrenic patients’ failure to suppress saccades may depend upon the type of activity that is required at the onset of the visual stimulus. Hutton et al. (2002) observed no deficits when schizophrenic patients were required to inhibit reflexive saccades in a fixation with distractors task. In contrast, when the subjects were required to generate an antisaccade rather than continue fixation, they produced significantly more reflexive saccades than the controls. The finding that when fixation is required schizophrenia patients’ inhibition is as efficient as that of controls is consistent with prior work (e.g., Gooding, Hendershot, & Grabowski, 2000) which indicates normal fixation in schizophrenia patients.
In a more recent investigation of schizophrenia patients’ fixation stability, Barton and colleagues (2008) observed that although a subset of schizophrenia patients had little difficulty maintaining fixation, many of the patients displayed difficulty maintaining fixation in a simple fixation with distractor task. The findings of Hutton et al. (2002) and Barton et al. (2008) can be reconciled if one considers that in the former study, the different tasks (i.e., antisaccade and fixation with distractor) were presented in blocks. In the Barton study, the antisaccade and fixation with distractor trials appeared pseudorandomly, possibly causing the task to be more difficult and revealing a subset of subjects with poor fixation ability. The increased difficulty unmasked a relationship between antisaccade performance and fixation ability. Indeed, Barton and colleagues observed a higher antisaccade error rate in patients with poor fixation stability.
Schizophrenics’ Performance on Scanpaths and Visual Search Tasks
When subjects were required to use visuospatial cues (arrows) to guide their sequence of scanning, Reischies et al. (1989) observed that schizophrenia patients produced a higher number of errors and displayed increased saccadic latency, relative to normals. When Gaebel, Ulrich, and Frick (1987) examined the visuomotor performance of partially remitted schizophrenia patients in a picture viewing task, they found two subgroups of patients who displayed opposite scanning patterns, both of which differed from that of healthy controls. Some patients engaged in minimal scanning, while other patients displayed extensive, though relatively inefficient, visual search behavior.
Kojima’s lab (Nakajima et al., 1988; Kojima, Potkin, Kharazmi, Matsushima, Herrera & Shimazono, 1989; Kojima et al., 1990) conducted a series of investigations of schizophrenia patients’ visual search performance, all of which replicated the earlier Gaebel et al. (1987) finding that the patients display poorer visual search performance than normal controls. Schizophrenia patients produce fewer exploratory eye movements when presented with a geometric figure to draw and then compare to two additional figures that differ slightly from the original stimulus. Schizophrenia patients also display a more limited area of inspection relative to normal controls and nonschizophrenic psychotic patients such as methamphetamine psychotic patients (Kojima et al., 1990). This finding of decreased responsive visual search behavior has been replicated in remitted schizophrenia patients as well as acutely ill patients and remitted patients (Kojima et al., 1990). Moreover, schizophrenia patients and schizotypal personality disordered patients do not differ in terms of their exploratory eye movements (Tsunoda et al., 2005), suggesting that this anomaly is also observed in the schizophrenia spectrum. In contrast, schizophrenia patients display significantly fewer exploratory eye movements than patients outside the schizophrenia spectrum, such as depressive patients (Kojima et al., 1992; Kojima et al., 2001; Matsushima et al., 1998). In summary, schizophrenia patients are generally able to produce visually guided saccades, particularly when the task is simple. Overall, their predictive saccade performance is also within normal limits, though reaction times may be increased. The most consistent finding in the saccadic literature concerns schizophrenia patients’ antisaccade task deficits. This appears to be a nearly ubiquitous finding; there is also compelling evidence indicating that schizophrenia patients’ antisaccade task deficits are a temporally stable trait. Schizophrenia patients also display aberrant performance on memory-guided saccade tasks. Although studied less frequently, schizophrenia patients’ performance on scanning and visual search tasks reveals significant differences from healthy controls and other patients outside the schizophrenia spectrum.
3A. Symptom Correlates of Schizophrenia Patients’ saccadic abnormalities
Some investigators have explored the role of symptoms on antisaccade task performance in schizophrenia patients. There have been a few reports of significant associations between negative symptoms, as measured by either the SANS scale or the PANSS, and suppression errors on antisaccade tasks (Ettinger et al., 2004a; Ettinger et al, 2006; Muller et al., 1999; Tien et al., 1996). However, schizophrenia patient groups classified according to predominance of negative symptoms do not differ significantly in terms of antisaccade accuracy (Nkam et al., 2001; Rosse et al., 1993). In contrast, when patients are grouped according to the deficit versus non-deficit distinction, differences in latency of successful antisaccades emerge (Nkam et al., 2001; Thaker et al., 1989). In both comparisons, the deficit patients showed significantly increased latency of antisaccades relative to the nondeficit group. Consistent with these findings, Louchart de la Chapelle et al. (2005) observed an association between antisaccade latency and negative symptoms, as measured by the PANSS negative subscale. Positive symptom ratings have been significantly associated with prosaccade gain (Ettinger et al., 2006).
Results of a 3-year follow-up investigation by Gooding, Mohapatra, and Shea (2004) indicated that antisaccade performance in schizophrenia patients is relatively stable, despite changes in clinical status. These findings, along with observations of antisaccade impairments in clinically remitted (Curtis, Calkins, Feil, & Iacono, 2001) or partly remitted (Reuter et al., 2005) schizophrenia patients suggests that if clinical symptoms explain any of the variance in antisaccade performance, it is likely to be a very small part.
3B. Saccadic performance in schizotypal populations
Studying saccadic performance in individuals with schizotypal traits and/or symptoms provides an indirect means of addressing the issue of symptom correlates of antisaccade abnormalities in schizophrenia, as well as the issue of whether the antisaccade task deficits precede the overt manifestation of schizophrenia. Investigators have identified individuals with schizotypal traits using a variety of methods, including the psychometric method, namely, classifying individuals on the basis of their scores on self-report measures of schizotypal traits (Gooding, 1999; Klein, Brugner, Foerster, Muller, & Schweickhardt, 2000; O’Driscoll, Lenzenweger, & Holzman, 1998). Some investigators (e.g. Wonodi et al., 2006) have recruited schizotypal individuals using advertisements and following up with clinical interviews in order to yield a group of individuals meeting diagnostic criteria for schizotypal personality disorder. Others (Brenner et al., 2001) have studied patients diagnosed with schizotypal personality disorder or individuals presenting with subsyndromal or prodromal symptoms of psychosis (e.g. Nieman et al 2007). Findings based on these different sampling strategies have been somewhat mixed.
To date, there are no reports of prosaccade deficits in schizotypal individuals, though Ettinger et al. (2005) observed an association between prosaccade spatial error and positive schizotypy. Nearly all of the investigations of psychometric schizotypes (i.e., individuals possessing schizotypal traits identified on the basis of their psychometric profiles) have found antisaccade deficits using the standard version of the antisaccade task. Investigators (Ettinger et al., 2005; Gooding, 1999; Gooding, Shea, & Matts, 2005; Holahan & O’Driscoll, 2005; O’Driscoll, Lenzenweger, & Holzman, 1998; Smyrnis et al., 2003) have observed elevated error rates in individuals with positive symptom schizotypy, as defined by the Perceptual Aberration scale (Chapman, Chapman, & Raulin, 1983) and/or Magical Ideation scale (Eckblad, Chapman, & Chapman, 1983). It is noteworthy that not all of the studies examining the association between positive schizotypal symptoms and antisaccade error rates compared groups with extreme scores; similar results were found in studies (Ettinger et al. 2005; Smyrnis et al. 2003) that examined antisaccade performance as a function of continuously distributed schizotypy scores. Interestingly, in their study of approximately 1200 Air Force recruits, Smyrnis et al. (2003) noted that the effects of state-dependent factors of anxiety and depression were more significantly related to antisaccade performance than schizotypy. The sole negative finding of group differences between high- and low-scoring individuals on a schizotypy questionnaire (ie., Raine’s Schizotypal Personality Questionnaire) may be attributable to an inadequate sampling strategy whereby an insufficiently extreme cutoff was used to identify the high schizotypy group (Klein et al., 2000).
Negative schizotypal traits, such as social and physical anhedonia, are conceptualized as related to the negative symptoms of schizophrenia. Social anhedonia (Gooding, 1999; Gooding, Shea, & Matts, 2005) appears to be significantly associated with performance on antisaccade tasks, though physical anhedonia does not (Holahan & O’Driscoll, 2005). In a five-year follow-up of schizotypal individuals and controls (Gooding et al., 2005), both schizotypal groups made more reflexive saccades than controls, though the subjects characterized by social anhedonia produced significantly more errors than the positive schizotypy subjects. Moreover, Gooding et al. (2005) noted, although antisaccade task performance at the baseline (Time 1 testing) predicted most of the variance in subjects’ follow-up performance, their self-reported social anhedonia scores at Time 1 accounted for a proportion of the variance over and above their initial saccadic performance.
Brenner et al (2001) reported that patients diagnosed with DSM-IV schizotypal personality disorder displayed normal saccadic performance on both antisaccade and memory-guided tasks, though there was some evidence of inhibitory deviance in a subgroup of SPD subjects. It is possible that the overall negative findings in the Brenner et al (2001) investigation are attributable to the less demanding nature of the antisaccade task that was used. This interpretation is consistent with the fact that in other prior studies of schizotypy that employed the overlap version of the antisaccade task (Holahan & O’Driscoll, 2005; Klein et al., 2000; Larrison et al., 2000) the schizotypal group did not differ from the healthy controls. Larrison et al. (2000) noted that the schizotypal group only displayed significantly greater errors on the gap version of the antisaccade task, suggesting that the task needed to be sufficiently challenging in order to elicit a deficit in this nonpatient group. Another group (Wonodi et al., 2006) examined the performance of a small group of individuals with subthreshold DSM-III-R Cluster A (paranoid, schizoid, or schizotypal) personality disorders on a standard antisaccade task. These individuals did not differ significantly from healthy controls in terms of their antisaccade error rates. It is unclear whether the negative finding is due to the small sample size (i.e., less than 15 subjects), the heterogeneous composition of their group of putatively schizotypal individuals, or both factors. Overall, there appears to be an association between schizotypy, the latent personality organization that all true carriers of the schizophrenia diathesis are hypothesized to possess (Meehl, 1962; Gooding & Iacono, 1995; Beauchaine, Lenzenweger, Waller, 2008), and antisaccade impairments. It is not clear, however, whether the positive aspects of schizotypy, which are conceptualized as related to the positive symptoms of schizophrenia, or certain traits of negative schizotypy, i.e., social anhedonia, are more strongly correlated with elevated rates of reflexive errors on antisaccade tasks. If antisaccade task deficits are an indicator of psychosis-proneness, it is plausible that both positive and negative aspects of psychosis-proneness may be associated with antisaccade task performance. Taken together, these research findings suggest that antisaccade task deficits may be associated with schizotypal traits, though they are independent of the clinical manifestation of overt psychotic symptoms
Further support comes from a study of antisaccade performance in prodromal individuals. Nieman et al. (2007) conducted a comparison of prodromal patients (classified as “ultra high risk” for psychosis), schizophrenia patients, and nonpsychiatric controls. The group of patients was deemed to be at “ultra high risk” (UHR) for psychosis on the basis of any of the following: genetic risk accompanied by a decline in functioning; the presence of attenuated psychotic symptoms; or a history of brief, time limited intermittent psychotic symptoms. The schizophrenia patients made significantly more errors than the UHR group, who, in turn, produced significantly more errors than the controls. Furthermore, the results (Nieman et al., 2007) indicated a trend towards a higher antisaccade error rate at baseline in the UHR patients who later transitioned to psychosis, relative to those who did not make the transition to psychotic illness. These findings suggest that antisaccade task deficits are most likely present prior to the onset of psychosis. Overall, findings based on schizotypal samples are similar to those derived from the schizophrenia studies. Most of the studies indicate that schizotypal individuals produce normal visually guided saccades and are more likely to show antisaccade task deficits than nonschizotypal individuals.
4. Neural Correlates of Saccadic Performance in Schizophrenia
In humans1, evidence from functional imaging studies (Melamed & Larsen 1979), electrophysiological studies (Evodokimidis et al., 1996; Klein, Heinks, Andresen, Berg, & Moritz, 2000), lesion studies (Guitton et al., 1985; Pierrot-Deseilligny, 1994; Gooding, 1997; Gooding, Iacono, & Hanson, 1999), and clinical studies (c.f. Pierrot-Desilligny, 1991; Gooding et al., 1997) suggest that the antisaccade task makes significant demands on the prefrontal cortex, particularly, its dorsolateral aspects. Readers are referred to the paper by McDowell and colleagues (2008) in this volume for a more detailed discussion of this topic.
Structural neuroimaging studies offer clues regarding the neural correlates of schizophrenia patients’ saccadic abnormalities. Increased antisaccade error rates have been associated with structural frontal lobe atrophy using CT ( Fukushima et al., 1988). In a voxel-wise analysis, Bagary et al. (2004) observed that greater error rates were associated with reduced grey matter volume in the right medial superior frontal cortex in their first-episode psychosis sample, though in the Ettinger et al (2004a) volumetric study of first-episode psychosis, the patients differed from the healthy controls by displaying an absence of correlation between premotor cortex volume and antisaccade error rate. In terms of saccadic latency, Schulze et al (2006) found an association between smaller prefrontal lobe volume and longer latency of correct antisaccades in both chronic schizophrenia patients and controls. Using diffusion tensor imaging (DTI, see glossary), Manoach et al (2007) observed that reduced fractional anisotropy in the white matter underlying the anterior cingulate cortex (see glossary), FEF (see glossary) and right posterior parietal cortex was associated with longer saccadic latencies in schizophrenia. Voxel-based structural MRI analysis revealed that schizophrenia spectrum patients’ decreased exploratory search behavior was significantly correlated with reduced gray matter in right fronto-parietal regions, namely, FEF, SEF (see glossary), PEF (see glossary), and inferior frontal cortex (Tsunoda et al., 2005).
Functional neuroimaging studies provide empirical support for the hypothesis that antisaccade task deficits displayed by schizophrenia patients has fronto-striatal neural substrates. Some studies contrast the regions activated during control tasks (e.g. simple saccadic refixation tasks) with those activated during antisaccade tasks. fMRI (see glossary) has also been useful in demonstrating that schizophrenia and control groups do not differ in terms of the degree of BOLD signal increases in regions associated with basic saccade generation, i.e., posterior parietal cortex, FEF, and SEF (McDowell & Clementz, 2001). Although healthy controls show significantly increased BOLD signal in the prefrontal cortex during their antisaccade task performance, schizophrenia patients do not (McDowell & Clementz, 2001; McDowell et al. 2002). These two studies conducted by McDowell and Clementz (2001) suggest that while the basic saccade generating circuitry is functionally intact among schizophrenia patients, the circuitry underlying antisaccade performance task is disturbed.
Using single photon emission tomography (SPET), Crawford and colleagues (1996) noted that patients with elevated error rates showed decreased activation in anterior cingulate, insula, putamen, and globus pallidus relative to patients with normal antisaccade performance. Raemaekers et al (2002), using functional magnetic resonance imaging (fMRI) noted that schizophrenia patients activated frontal and parietal cortex normally during an antisaccade task, though they failed to activate the striatum to the same extent as healthy controls. In a recent study (Polli et al 2008), investigators used event-related fMRI and an antisaccade paradigm with concurrent monitoring of eye position. They discovered that reduced activation in the anatomical components of two ACC circuits, namely the dorsal ACC circuit and the rostral ACC circuit was related to increased error rate in the schizophrenia patient group. The association between reduced activation in the ACC and schizophrenia patients’ persistence of reflexive errors is interesting, because the ACC is theorized to play a role in error processing.
Possible Mechanisms Underlying Antisaccade Task Deficits in Schizophrenia Patients
There have been several interpretations of schizophrenia patients’ antisaccade task deficits. In the following section, we summarize various research efforts to identify mechanisms underlying the observed impairments. Perhaps the most parsimonious interpretation of patients’ antisaccade deficits is that they reflect a general oculomotor deficit. However, given schizophrenia patients’ ability to perform within normal limits on simple saccadic refixation tasks, their abnormal performance on antisaccade tasks cannot be attributed to an overall defect in generating saccades. Nearly all of the other explanatory accounts for schizophrenia patients’ antisaccade abnormalities are focused on their executive functioning deficits. Executive functioning includes the following processes: focusing attention, inhibition, task management, set shifting, planning and implementing behavior, and monitoring and updating (Lezak, 1983; Smith & Jonides, 1999). These explanatory accounts are plausible, given that one of the most characteristic features of schizophrenia patients’ cognitive deficits is their impaired executive functioning. To date, accounts of schizophrenia patients’ antisaccade task deficits have included: impairments in attentional focus; impaired inhibitory control; impairments in the implementation of inhibition; perseveration of sets; perseveration of response; working memory impairments; goal neglect; and an inability to generate a voluntary action. In our discussion and evaluation of these accounts, supportive data, particularly pertaining to neurocognitive correlates of patients’ antisaccade task performance, will be cited.
Impairments in Attentional Focus
Researchers have hypothesized that schizophrenia patients’ antisaccade task deficits are secondary to their attentional impairments and inability to filter out irrelevant information or redirection of attentional engagement (Maruff et al., 1996, 1998). The results of Tendolkar et al. (2005) provide support for the former interpretation. These investigators recorded ERPs while subjects performed prosaccade and antisaccade tasks. They compared healthy controls and schizophrenia patients in terms of the P100 component of the ERP, which has an enhanced amplitude in response to suppressing unattended stimuli. The controls displayed a larger P100 for antisaccades relative to prosaccades, though the schizophrenia patients failed to do so; this suggests that the patients, who produced significantly more errors on the antisaccade task, were less successful in suppressing the irrelevant stimulus attributes in the antisaccade task (Tendolkar et al., 2005).
Researchers have also suggested that attentional impairments in sustained attention may underlie patients’ antisaccade task deficits. Neuropsychological test findings indicating an association between performance on measures of attentional functioning such as the CPT and Trails A and antisaccade performance in schizophrenia patients support this notion. Radant et al. (1997) noted that antisaccade errors correlated significantly with performance on Part A of the Trail Making Test in schizophrenia patients, but not in the healthy controls. Although there was suggestive evidence of an association between Part B of the Trail Making Test and antisaccade performance, the correlation failed to reach statistical significance. Performance on the CPT, a measure of sustained attention, explains a significant amount of the variance in schizophrenia patients’ antisaccade task performance accuracy (Donohoe, et al., 2006). The neuropsychological data are consistent with oculomotor data recently reported by the Sweeney group (Reilly et al., 2008). Reilly et al. found an association between the ability to focus attention, as indexed by the difference in prosaccade latencies between the no-gap versus overlap trials, and executive control on the antisaccade task, as measured by error rates. These results indicated that in schizophrenia patients, decreased attentional focus on central cues was associated with increased antisaccade errors.
Working Memory Impairment
Working memory involves the following processes: temporary storage of information, manipulating the stored information, and using it to guide subsequent behavior (Goldman-Rakic, 1987, 1994). Working memory components of the antisaccade task include retaining the task instructions, forming an internal representation of the target stimulus and its location, and using the task instructions to guide subsequent oculomotor behavior, i.e., suppression of the reflexive saccade and generation of a voluntary saccade to the mirror image of the target stimulus. Several researchers have asserted that schizophrenia patients’ antisaccade task deficits reflect their working memory impairments (Gooding & Tallent, 2001; Hutton et al., 2004). Findings based upon neurocognitive testing which indicate significant correlations between working memory performance and antisaccade task performance buttress support for the hypothesis that schizophrenia patients’ increased frequency of directional errors reflects an impairment in working memory.
As noted earlier, schizophrenia patients’ WCST performance is correlated with their antisaccade task performance (Crawford et al., 1995; Karoumi et al., 1998; Louchart de la Chapelle et al., 2005; Rosse et al., 1993; Tien et al., 1996). The WCST has a significant working memory component; indeed, some (Park, 1997; Gold et al., 1997) assert that the working memory component of the WCST is the rate limiting step that determines schizophrenia patients’ task performance. Most investigations (Donohoe et al., 2006; Gooding & Tallent, 2001; Hutton et al., 2004; Nieman et al., 2000) that directly examine the relationship between schizophrenia patients’ antisaccade task performance and their working memory performance indicate a relationship, though Snitz and associates (1999) failed to do so. The relationship between spatial working memory and antisaccade task performance is present in first-episode schizophrenia patients (Hutton et al., 2004; Nieman et al., 2000) as well as more chronic patient groups (Donohoe et al., 2006; Gooding & Tallent, 2001). Clearly, then, the working memory aspect of executive functioning is relevant for antisaccade performance (Hutton et al., 2004).
Impairments in Inhibitory Control
Some investigators assert that schizophrenia patients’ antisaccade deficits could be better explained in terms of an inhibitory control deficit2. In the gap prosaccade task, attention is already disengaged from the central target prior to the cue appearance; as such, the gap condition of the prosaccade task potentiates disinhibition. Investigators have interpreted schizophrenic patients’ performance on the gap saccade paradigm as an indicator of their inhibitory control. Schizophrenia patients show a greater decrease in SRT compared to healthy controls (Levy et al., 1998; Sereno & Holzman, 1993); the fact that patients produce more express (short latency) saccades relative to controls is consistent with their having a dysregulated inhibitory system. In the overlap condition, in which the central target remains (overlaps) with the appearance of the peripheral cue, response inhibition is facilitated. In this condition, when schizophrenia patients were given a greater amount of time to inhibit a reflexive saccade, their error rates normalized (Levy et al., 1998). Levy and colleagues interpreted patients’ greater dependence on time to disengage from the central target as indicative of an inhibitory dysregulation.
Neurocognitive test findings also suggest that inhibitory deficits may contribute to, and/or account for, schizophrenia patients’ elevated antisaccade error rate. Levy et al. (1998) noted that on the WCST, perseverative errors, which reflect a failure of inhibition, are associated with antisaccade error rate. In contrast, the number of categories achieved, an index of WCST performance that does not require an inhibitory process, does not correlate with antisaccade error rate. After noting that performance on the go/no go task accounted for more than twice as much variance in antisaccade performance as the CPT performance, Donohoe et al. (2006) concluded that antisaccade task performance in schizophrenia reflects impairments in inhibitory control, rather than more general attentional impairments.
While the aforementioned studies indicate that schizophrenia patients display failure of inhibition, other experimental evidence suggests that impairments in inhibitory control cannot solely account for patients’ antisaccade task deficit. For example, Hutton et al (2002) demonstrated that patients were able to suppress a reflexive saccade to a distractor in a fixation task but were unable to do so in an antisaccade task, suggesting that their failure of inhibition is task dependent. An investigation of 81 schizophrenia patients revealed no association between antisaccade performance and P50 sensory gating, another measure of inhibition (Louchart de la Chapelle et al., 2005). Similarly, Kumari et al. (2005) reported findings from a series of studies indicating that there was no significant relationship between prepulse inhibition and percentage of correct antisaccades in schizophrenia patients.
Difficulties implementing inhibition of errors
Barton’s group (2008) asserts that the antisaccade errors manifest as a failure in implementing inhibitory control, rather than an inhibitory deficit. In their comparison of chronic outpatients with healthy controls on a series of fixation, prosaccade, and antisaccade trials, they found that fixation instability was correlated with antisaccade errors. This association lent support to their assertion that a problem with implementing inhibition played a contributing role in the high antisaccade error rate of schizophrenia patients. This later refinement of their earlier hypothesis is plausible; continuing to accelerate (a perseveration of a specific response) may be an inappropriate response, but the problem is compounded when the driver fails to engage the brake pedal when necessary (implement inhibition).
Other investigators suggest that in addition to deficits in implementing inhibition, schizophrenia patients with antisaccade task deficits may have a more specific problem with novel response generation. Rather than being due to difficulty in generating novel responses, an alternative hypothesis is that schizophrenia patients are unable to generate volitional acts in situations in which routine actions are inappropriate (Frith, 1992). This inability to generate voluntary actions in this context would account for deficits in the inhibition of the prepotent response when triggered by environmental stimuli such as the antisaccade task (Frith, 1992; Reuter et al., 2005). In this way, another executive functioning deficit is proposed to contribute to, if not entirely account for, schizophrenia patients’ antisaccade task deficit.
Goal Neglect
Nieuwenhuis et al. (2004) argue that different findings in the saccadic literature are attributable to the extent to which different tasks elicit goal neglect, defined as failure to fully or consistently focus attention on task requirements despite understanding them. Nieuwenhuis and colleagues used an adaptation of the antisaccade task, in which they administered cued target discrimination tasks to schizophrenia patients and controls. In these tasks, the instructions only mentioned the need to discriminate between the targets and indicate their choice using a finger press. The instructions did not explicitly refer to the need for making instrumental saccades, despite there being two cue conditions (i.e., pro-cue or anti-cue). When the cued location and the actual spatial location of the stimulus were in conflict, the schizophrenia patients’ discrimination error rates were significantly higher than the healthy controls. The finding that patients’ voluntary components of their procue performance was similarly impaired as their anticue performance is consistent with the interpretation that schizophrenia patients’ goal neglect adversely affects the endogenous component of their task performance. Nieuwenhus and colleagues apply the Duncan (1995) construct of goal neglect to account for schizophrenia patients’ antisaccade task deficits. This model of antisaccade task deficits provides an account for why elevated errors are consistently observed in schizophrenia patients regardless of the relative difficulty of the task version; due to the nature of the antisaccade task, the subject must maintain an internal representation of the goal in order to override the prepotent response. Reuter and Kathmann’s (2004) hypothesis of inefficient goal dependent activation of motor actions is quite similar to the Nieuwenhuis et al. account, though it is more comprehensive in that it explicitly takes inhibitory deficits into consideration.
In our view, goal neglect/goal activation deficits can be considered a specific mechanism that is wholly consistent with a working memory deficit, because the individual is failing to use the on-line information, specifically, the task requirements or the task goal, to guide subsequent behavior. Indeed, a requisite component of normal antisaccade task performance would be the ability to maintain an internal representation of the task goal over a delay to guide one’s saccadic behavior (i.e., working memory). A deficit in goal activation may therefore be a manifestation of impaired working memory. Indeed, Reuter, Rakusan, and Kathmann (2005) concur that the Gooding and Tallent (2001) working memory account for schizophrenia patients’ antisaccade task deficit is consistent with either a goal neglect or goal activation deficit hypothesis.
Perseveration of Response
Some investigators hypothesized that schizophrenia patients’ antisaccade task deficits might reflect difficulty switching sets; this is certainly plausible, given observed associations between patients’ WCST performance and their antisaccade task performance (Crawford et al., 1995; Karoumi et al., 1998; Louchart de la Chapelle et al., 2005; Rosse et al., 1993; Tien et al., 1996). Subjects might encounter greater difficulty achieving subsequent WCST categories after being inferring that task requirement was to sort by a specific category (e.g. color), much as they would encounter difficulty producing antisaccades after performing prosaccades, especially if the trials were interspersed. However, Barton et al. (2002) hypothesized that task switching and antisaccade performance were independent functions in both schizophrenia patients and controls. In their investigation, schizophrenia patients and controls showed a similar pattern of the effects of task switching on prosaccades and antisaccades. These data supported the Barton et al. (2002) hypothesis that task switching and antisaccade performance are independent.
Subsequently Barton and colleagues observed that in schizophrenia patients, an antisaccade delays saccades two trials later, though this same effect was not observed in healthy controls. The schizophrenia patients displayed an abnormal persistence in the state of the saccadic response system, especially when induced by recent antisaccades, resulting in perseveration. Thus, the investigators reasoned that schizophrenia patients’ elevated antisaccade error rate reflects a perseveration of specific saccadic responses, rather than perseveration of task sets (Barton et al., 2005). In their attempts to identify the mechanisms underlying schizophrenia patients’ antisaccade task impairments, researchers have systematically varied parameters of the saccadic tasks in order to parse different components and/or adapted the saccadic tasks. Others have included neuropsychological measures and psychophysiological tasks in their experimental armamentarium along with saccadic tasks in order to identify correlates of schizophrenia patients’ antisaccade task performance. At present, we can rule out the notion that these patients’ antisaccade task deficit reflects a generalized oculomotor impairment. However, as discussed above, several other viable interpretative accounts remain.
Saccadic Performance in Other Psychiatric Patient Populations
An increasing number of studies have addressed saccadic performance in other psychiatric patient populations. In the next section, findings from these studies will be reviewed. Data regarding comparisons between other psychiatric patient groups and schizophrenia patients will be discussed as well.
1. Saccadic Performance in Mood Disordered Patients
In contrast to the body of literature on saccadic eye movements in patients with schizophrenia and schizophrenia-spectrum disorders, there is relatively little research on saccadic task performance in mood disordered patients. In early investigations of saccadic performance, patients with mood disorders were regarded primarily as psychiatric control subjects and were therefore combined into a single group. Mixed groups of affective disordered patients do not differ from healthy controls in terms of saccadic accuracy, as measured by error rate, on prosaccade tasks (Sereno & Holzman, 1993; Curtis et al., 2001), though acutely psychotic affective patients may show lower prosaccade gain (Curtis et al., 2001). Mixed groups comprised of bipolar and major depressive patients do not differ from nonpsychiatric controls in terms of saccadic reaction time on saccadic refixation tasks (Curtis et al, 2001; Sereno & Holzman, 1993).
Sereno and Holzman (1995) observed elevated antisaccade errors in an affective group composed of mostly (73%) bipolar patients. Katsanis et al. (1997) found increased antisaccade errors in a mixed group of inpatients with either major depression with psychotic features or bipolar disorder with psychotic features. Similarly, Curtis et al. (2001) observed a significantly elevated error rate among acutely psychotic mood disordered patients, as well as prolonged latencies during correct antisaccade trials, relative to the nonpsychiatric comparison group. These studies suggest that antisaccade task deficits may be associated with mood disorder with psychotic features. Patients with schizophrenia produced significantly greater antisaccade errors than the nonschizophrenic psychotic patients in the Curtis et al. (2001) investigation, though not in other studies (Katsanis et al., 1997; Sereno & Holzman, 1995).
2. Saccadic Performance in Major Depressive Disorder
Not only have investigators begun to test mood disordered patient groups separately, rather than comparing a mixed group of “affective patients” to nonpsychiatric controls, but even more promising, they are attempting to compare different subtypes of patients within a given diagnostic category. Overall, patients with major depressive disorder display normal accuracy rates on visually guided saccade tasks (Crevits et al. 2005; Done & Frith, 1989; Mahlberg et al., 2001) as well as voluntary refixation tasks (Crevits et al, 2005). Sweeney et al. (1998) reported that depressive patients showed reduced saccade accuracy on a visually guided saccade task; however, their study sample consisted of a group of unmedicated depressed inpatients, 11 (38%) of whom had psychosis. The data regarding saccadic latencies on the prosaccade tasks are mixed. Done and Frith (1989) reported that drug-free depressed patients showed normal saccade latencies to suddenly appearing peripheral targets, though they displayed a prolonged latency of voluntary saccades to predictable targets. Mahlberg et al. (2001) also observed that depressed patients, who had been drug free for at least 7 days, had longer reaction times than controls. Yet in the Sweeney et al. (1998) investigation of unmedicated inpatients with major depression, the subjects displayed normal latencies and velocities for voluntary saccades.
Consideration of a series of studies by Winograd-Gurvich and colleagues (2006a, 2006b) helps to reconcile these seemingly inconsistent findings. Winograd-Gurvich et al. (2006a) grouped the depressive outpatient sample according to whether or not they met diagnostic criteria for the melancholic subtype. The investigators found performance differences between the melancholic depressives and their non-melancholic counterparts, whereby the latter group performed similarly to healthy controls on most saccadic tasks. In contrast, melancholic depression was associated with longer latencies, hypometric primary saccades during the predictable saccade paradigm, and difficulty increasing peak velocity as target amplitudes increased. In a subsequent investigation, Winograd-Gurvich et al. (2006b) administered self-paced saccade tasks in order to elicit prosaccades as well as two saccadic ‘oddball’ tasks. In the ‘oddball’ tasks, at pseudorandom occasions during a saccadic refixation task, an oddball target appeared at a location in the same direction as the expected target location but to a different extent (e.g., 15° to the right instead of 7.5° to the right). The purpose of the saccadic oddball task was to test individuals’ ability to reprogram saccades. Neither the melancholic nor non-melancholic depressive patients differed from the controls in terms of accuracy or latency on the self-paced saccade tasks. However, the melancholic depressed group was less accurate than the other groups on the reprogramming saccade tasks. Moreover, the investigators noted a significant association between severity of melancholic symptoms and latencies for saccades requiring on-line reprogramming of saccade direction. Given the role of the basal ganglia (see glossary) in the inhibition and selection between competing responses, the findings of Winograd-Gurvich et al. (2006b) are consistent with the notion of striatal involvement in the underlying pathophysiology of melancholic depression.
Investigations of antisaccade task performance in patients with major depression indicate that mild or moderately depressed patients display normal rates of suppression errors ( Fukushima et al., 1990), while acutely ill, unmedicated depressive inpatients display elevated error rates (Crevits et al., 2005; Sweeney et al. 1998) and increased antisaccade response latency (Crevits et al., 2005). In a study of unmedicated depressed inpatients, many (38%) of whom had psychosis, Sweeney and colleagues (1998) saw evidence of reduced accuracy for memory-guided saccades, namely, less accurate final resting eye position as well as increased saccade duration. Taken together, these findings suggest that antisaccade impairments in major depression may be state-related.
Studies of exploratory eye movements in depressive patients reveal that they make fewer eye movements than healthy controls when they are acutely ill though they do not differ from controls when they are in remission (Kojima et al., 2001). This suggests that, like antisaccade task deficits, any abnormalities in the visual search behavior of individuals with major depression, are unlikely to be a stable deficit.
3. Saccadic Performance in Bipolar Mood Disorder
On visually guided saccade tasks, individuals with bipolar disorder do not differ from healthy controls in terms of accuracy or response latency (Crawford et al., 1995; Gooding et al., 2004; Tien et al., 1996). Based largely on inpatient samples, investigations of antisaccade task performance in bipolar disordered patients reveal increased errors (Martin et al., 2007; Tien et al., 1996). Typically, studies based on psychotic bipolar outpatients (Crawford et al., 1995: McDowell & Clementz, 1997) indicate that most patients fall within the middle of the range of the nonpsychiatric controls. Although Gooding and Tallent (2001) observed elevated suppression errors among a sample of clinically stabilized outpatients, a follow-up study based on a subset of those subjects (Gooding et al., 2004) indicated that antisaccade performance is not temporally stable in bipolar disorder. These findings suggest that the antisaccade task deficits displayed by bipolar patients may be reflective of state-related factors. Most comparisons (Crawford et al., 1995; Gooding & Tallent, 2001; Tien et al., 1996) indicate that schizophrenia patients produce significantly more antisaccade errors than the bipolar patients (but see Martin et al., 2007).
Bipolar patients generate accurate saccades to remembered targets on oculomotor delayed response tasks (Crawford, 1995; Park & Holzman, 1992, 1993). These patients display prolonged response times to remembered targets in some reports (Park & Holzman, 1993) but not others (Crawford, 1995; Park & Holzman, 1992). Park and Holzman (1992) also demonstrated that bipolar patients have a delayed initiation of saccades made on command towards continuously illuminated targets. Together, these data imply that individuals with bipolar mood disorder may have inhibition deficits, as evidenced by their impaired antisaccade task performance, though their working memory appears generally intact, as evidenced by the overall accuracy of their memory-guided saccades.
4. Saccadic Performance in Borderline Personality Disorder
To date, there has only been one investigation of saccadic performance in individuals with borderline personality disorder. Grootens et al (2008) studied antisaccade performance in 32 borderline patients (20 of whom had transient psychotic symptoms),21 recent onset schizophrenia patients, and 25nonpsychiatric controls. The schizophrenia group made more inhibition (antisaccade) errors and had longer saccade initiation times than the borderline group or the controls. The borderline patients also produced more inhibition errors than the controls. Interestingly, the borderline personality disordered patients with psychotic-like symptoms made more antisaccade errors than the borderline patients without transient psychotic symptoms. The borderline patient group did not show any deviance in terms of antisaccade response latency, nor was there any correlation between the anticipatory errors on the saccadic task and the inhibitory errors on the antisaccade task. The disinhibition that the borderline personality disordered patients display on the antisaccade task is consistent with the impulsivity that characterizes their interpersonal behavioral responses.
The study findings from Grootens et al (2008) are intriguing in that the borderline personality disordered patients with transient psychotic symptoms showed a similar error rate on the antisaccade task as the schizophrenia patients, whereas the other borderline patients without psychotic-like symptoms showed a lower, albeit still aberrant, error rate. While this may suggest that the antisaccade errors may be an indicator of a liability towards psychosis, these findings are also consistent with a dysfunctional frontolimbic network being implicated in the underlying pathophysiology of borderline personality disorder (Brendel, Stern, & Silbersweig, 2005).
Saccadic Performance in ADHD
Relatively few investigations of saccadic performance in Attention Deficit Hyperactivity Disorder (ADHD) examine adults. Error rates in prosaccade tasks are similar across ADHD patients and healthy control populations (Carr et al., 2006; Fiefel et al 2004; Munoz et al., 2003). Findings regarding saccadic latency differences between ADHD patients and healthy controls vary. For example, Munoz et al. (2003) found that the latency of visually-guided prosaccades is longer and more variable in patients than in healthy controls regardless of whether the prosaccade is made within the context of an overlap condition or a gap condition. In contrast, Ross et al. (2000) saw no group differences in latency in visually-guided prosaccades and Fiefel et al. (2004) observed a trend whereby patients with ADHD had shorter latencies. However, ADHD patients consistently display normal saccade amplitudes (Fiefel et al., 2004; Munoz et al., 2003).
On predictive saccade tasks, ADHD patients demonstrate evidence of learning, as reflected by reduced reaction time, as well as healthy controls (Fiefel et al., 2004). Although the accuracy of the predictive saccades is statistically indistinguishable from healthy controls, the duration of these saccades is significantly increased. It is unclear why the duration of the movement would increase but the finding that ADHD patients can learn an oculomotor prediction task indicates that circuits responsible for sensory-motor learning are likely intact.
One of the most reliable findings in adults with ADHD is an increase in the number of directional errors in the antisaccade task (Carr et al., 2006; Fiefel et al., 2004; Munoz et al., 2003). Increased error rates in ADHD appear even when other symptoms are ameliorated (Carr et al., 2006). Furthermore, the increase in antisaccade directional errors appears larger in patients with the combined type of ADHD compared to those with the primarily inattentive form of ADHD (Carr et a., 2006) although both types of ADHD patients had more errors than controls. In spite of a sometimes longer saccade latency measured in ADHD patients compared to controls, the gap effect is generally present (Fiefel et al. 2004; Munoz et al. 2003), indicating that mechanisms of attentional disengagement or other processes underlying the gap effect appear to be intact in these patients.
Using the oculomotor delayed-response task, a comparative study of ADHD patients and schizophrenia patients revealed similarities in saccade deficits as well as important differences. Investigators (Ross et al., 2000) examined the percentage of trials with premature saccades during the delay period, as well as the latency and accuracy of saccades during subjects’ performance of a memory-guided prosaccade task. The ADHD patients’ saccade latencies were statistically indistinguishable from those measured in control subjects, whereas patients with schizophrenia had much longer latencies. In addition, the accuracy of the saccadic endpoint in the ADHD patients was similar to that in healthy controls. Ross et al. (2000) noted that both patient groups showed an increase in the number of premature saccades produced during the delay period; the finding that ADHD patients display difficulty suppressing saccades during the delay period has been replicated (Carr et al., 2006; Munoz et al., 2003). Thus, individuals with ADHD, like those with schizophrenia, have an apparent deficit in inhibitory control when a delay is imposed in a saccade task. Interestingly, further evidence shows that even when no saccade is required, simply maintaining fixation for extended periods is difficult for ADHD patients (Carr et al., 2006; Munoz et al., 2003).
Saccadic Performance in Tourette’s Syndrome
Research on saccadic performance in individuals with Tourette’s Syndrome (TS) is relatively sparse. Data suggests that TS patients’ reflexive prosaccade reaction times are normal or only slightly elevated (Farber, Swerdlow, & Clementz et al., 1999; Straube et al., 1997) and their saccadic durations are shorter (Farber et al., 1999). During a prosaccade gap task, Farber et al. (1999) noted that patients with TS showed more anticipatory saccades, though they did differ from healthy controls in terms of the proportion of “express saccades” that they produced.
Most of the investigations of TS patients have focused on antisaccade task performance, perhaps due to the fact that Tourette’s Syndrome is considered a hyperkinetic movement disorder that reflects a loss of the inhibitory control of voluntary movements thought to arise from an alteration in the dopaminergic tone of the basal ganglia (Albin&Mink, 2006). Surprisingly, findings regarding antisaccade performance among TS subjects are mixed. Some investigators (Dursun et al., 2000; Farber et al., 1999) report that TS patients show more directional errors in the antisaccade task than age- and sex-matched healthy controls, while others (LeVasseur et al., 2001; Straube et al., 1997) noted that there were no group differences. Through administering a battery of saccadic tasks, LeVasseur et al. (2001) gathered data that help reconcile these seemingly discrepant findings.
Individuals with Tourette’s Syndrome and healthy controls were administered immediate and delayed prosaccade tasks, immediate and delayed antisaccade tasks, and delayed and memory guided sequential saccade tasks. Similar to earlier reports (Straube et al., 1997), the investigators found increases in reaction time across all the saccadic tasks. Although the TS patients performed equally well as the healthy controls on the immediate antisaccade task, when they were required to suppress a reflexively evoked saccade over a delay, i.e., perform a delayed antisaccade task, they showed increased errors (LeVasseur et al., 2001). Based on the observation that the TS subjects’ increased directional errors required a delay in order to be elicited, LeVasseur et al. have suggested that the patients’ difficulty does not reflect a simple inability to suppress reflexive saccades. Rather, they asserted, TS patients have a more general inability to suppress a motor program.
Additional findings from the LeVasseur et al. (2001) study, based upon a memory guided sequential saccade task, provide empirical support for their alternative interpretation of the TS subjects’ elevated task errors. In the memory guided sequential saccade task, three separate target stimuli appeared in sequence while the subject fixated. Subjects kept fixating for an additional time period until the centrally-located fixation point disappeared. At that time, the subject was asked to repeat the sequence of saccades indicated by the previously flashed stimuli. LeVasseur et al. (2001) observed that the TS subjects experienced difficulty delaying saccades in the delayed and memory guided sequential saccade tasks, as reflected in an increased frequency of timing errors. When the patients made the timing errors, the errant saccade targeted the first of the three stimuli in the sequence, rather than the most recently occurring stimulus. Given that the timing errors in the memory guided sequential saccade tasks were usually triggered in the direction of the first saccade of the initial sequence, LeVasseur and colleagues concluded that TS patients’ saccadic deficits reflected an inability to delay the occurrence of a motor program, rather than an inability to suppress a reflexively evoked movement. Had they been unable to suppress the reflexive eye movement, the saccade would have targeted the last position in the sequence. The investigators asserted that the nature of the inhibitory deficits in Tourette’s Syndrome involves a more global inability to suppress a complex motor program.
The results of a second investigation provide insight into the nature of saccadic abnormalities in TS patients. In the task two pro-saccade trials occur and then two anti-saccade trials occur. This alternation between pro and anti-saccades continues at a predictable rate of two trials per condition. Additionally, the type of a trial is cued in advance at two different time intervals 200 and 1000ms (Mueller et al., 2006). In a second version of the task, the rate of the alternation occurs unpredictably and the pre-cue intervals also occur (Jackson et al., 2007). Not surprisingly, the presence of the pre-cue benefited the performance of both healthy control subjects and TS subjects. Surprisingly, on trials that switched from pro- to anti-saccades, the error rate measured in TS subjects was less than that measured in healthy subjects even though the reaction time was statistically indistinguishable. Taken together, these results argue strongly that TS patients are able to suppress movements even better than control subjects. It is not clear whether this enhanced performance reflects some kind of compensatory mechanism. Nevertheless, these new observations suggest that symptomatology in TS patients is more complicated than a loss of inhibition of reflexive movements. In particular, the finding that TS patients have enhanced control provides an interesting hypothesis to be tested that may reconcile the disparate results on saccade abnormalities in TS patients. Specifically, rather than co-morbidity, medication status, and age being the co-varying predictors of oculomotor performance, the varying levels of ability to exert descending control on movements may explain differences among patients.
Saccadic Performance in Obsessive Compulsive Disorder
There is a small but growing body of research that focuses on gaze fixation abnormalities in phobic patients (see Bar-Haim et al., 2007 for a recent review). In one intriguing study, social phobia patients displayed markedly different scanpath patterns, including reduced scanning of the interpersonal (facial expression) stimuli and fewer fixations to salient facial features (Horley, Williams, Gonsalvez, & Gordon, 2003). Overall, there has not been much study of saccadic eye movements in anxiety disorders.
There is a larger literature regarding saccadic performance in patients with obsessive compulsive disorder (OCD). The data suggest that OCD patients are unimpaired in terms of performance on visually-guided reflexive saccade tasks (Maruff et al., 1999; Nickoloff et al., 1991; Rosenberg et al., 1996; Spengler et al., 2006; Tien et al., 1992; van der Wee et al., 2006). Compared to healthy controls, patients with OCD show no significant differences in terms of saccadic gain or latency (Spengler et al., 2006), though one report (Rosenberg et al., 1996) indicated that saccade velocities were slower for larger saccades. On simple predictive visually guided tasks, OCD patients appear to display the predictive behavior as well as or even faster than healthy controls and schizophrenia patients (Nickoloff et al., 1991; Spengler et al., 2006).
There are only two reports of elevated antisaccade errors in OCD patients (Tien et al., 1992; Rosenberg, Dick, O’Hearn, & Sweeney, 1997). Rosenberg et al. (1997) observed that compared to healthy controls, nondepressed, unmedicated OCD patients displayed more antisaccade errors, though they showed normal accuracy and latency of memory saccades on the oculomotor delayed response task. Although investigators interpreted these findings as suggestive that OCD patients have a specific deficit in response suppression, others (c.f. Maruff et al., 1999; Nieuwenhuis et al., 2004) have criticized both these studies for an insufficient number of trials. A low number of trials can be problematic because they might not allow sufficient practice, thereby limiting task familiarity, and/or they may contribute to the task’s inadequate reliability.
Medication naïve OCD patients do not show increased errors on antisaccade tasks (Maruff et al., 1999; van der Wee et al., 2006). Maruff et al. (1999) observed that the OCD patients’ latency of both volitional prosaccades and antisaccades were slower than normal. Considered along with their normative antisaccade performance, Maruff and others have suggested that these patients do not have a response inhibition deficit, but rather, experience difficulty generating a saccade based on an internally represented goal. This hypothesized selective impairment would distinguish OCD patients from schizophrenia patients. Consistent with this alternative hypothesis, in other studies (McDowell & Clementz, 1997; Nieuwenhuis et al., 2004; Spengler et al., 2006), the performance of OCD patients do not differ significantly from that of healthy controls. Regardless of the interpretation of the circumscribed saccadic performance deficits observed in individuals with obsessive compulsive disorder, it appears that there is no correlation between their oculomotor performance and the severity of their symptoms (Maruff et al., 1999; Rosenberg et al., 1996). Taken together, the saccadic eye movement abnormalities in patients with Tourette’s, OCD and ADHD are similar in that visually-guided prosaccades are generally indistinguishable from healthy controls. In contrast, saccadic eye movements made in the absence of explicit sensory cues are often reported as deficient in these patients. In TS patients specifically, the extended study exploring sequences of saccades reveals that the underlying cause of the saccade abnormality may be a sophisticated deficit in controlling motor programs, not just suppressing reflexes. It will be interesting in the future to explore more sophisticated saccade tasks in patients with ADHD and OCD as have been done in Tourette’s Syndrome to determine whether similar underlying deficits are at play.
Discussion
This review illustrates the growing number of investigations of saccadic eye movements in adult psychiatric patients. A variety of oculomotor tasks designed to assess reflexive and volitional saccades have been administered in the hopes of discerning more about the processes that are impacted by the mental disorders and the pathophysiological mechanisms by which these processes are disrupted. In this section, we attempt to summarize findings across the disorders, and highlight the similarities and differences that have emerged from the literature. In the final section of our discussion, we consider the ways in which saccadic research can further the field of psychiatric and neurological research, and the potential that this work has in terms of clinical practice, genetics, and pharmacology.
Among schizophrenia patients, it appears that performance on simple visually guided saccadic tasks is normal, and patients display the gap effect. Their performance on predictive saccade tasks is also indistinguishable from healthy controls. In contrast, on complex prosaccade tasks, schizophrenia patients show marked deficits. They show elevated error rates on antisaccade tasks and memory-guided (oculomotor delayed response) tasks. Furthermore, schizophrenia patients make fewer exploratory eye movements on visual search tasks than either nonschizophrenic psychiatric comparison groups or healthy controls. It is noteworthy that individuals with schizotypal traits and/or schizophrenia spectrum disorders display a similar pattern of normal saccadic and aberrant antisaccade performance.
Despite the relative sparseness of data in nonschizophrenia patient populations, tentative conclusions can be drawn regarding distinct patterns of impairment. Overall, mood disordered patients display antisaccade task impairments, though these deficits may be state-related. The patients with more severe forms of illness, such as bipolar disordered disorder with psychotic features, or major depressive disorder of the melancholic subtype, are more likely to display elevated antisaccade task errors. Although bipolar patients show difficulties with antisaccade tasks, they display normal performance on ODR tasks. Melancholic depressive patients display problems with reprogramming saccadic tasks, and decreased exploratory search behavior as well as antisaccade task deficits. Among another group of patients with affective instability, namely, individuals with borderline personality disorder, patients with psychotic symptoms performed significantly worse on the antisaccade task than healthy controls.
Adults with ADHD display aberrant performance on fixation tasks, as well as antisaccade tasks and memory guided saccade tasks. Despite a few early reports to the contrary, it appears that patients with OCD perform within normal limits on antisaccade tasks. Some individuals with Tourette Syndrome produce excessive errors on delayed antisaccade tasks, and some have difficulty delaying saccades in memory-guided sequential saccade tasks. The data suggest that in the TS population, saccadic suppression varies according to the patients’ level of motoric control.
Most of these saccadic tasks are complex behavioral assays, involving multiple components, rendering it difficult to pinpoint exactly what process underlies the performance difference between the patients and controls. A technique of behavioral parsing (c.f. Levy et al., 1998) can allow one to infer likely sources of patients’ abnormal performance on the more complex saccadic tasks. For example, schizophrenic and ADHD patients show impaired performance on both antisaccade and oculomotor delayed response tasks, while bipolar patients show impairments only on the former task. In Table 1, we have parsed the different component processes involved in each of these saccadic tasks. As one can see, the two tasks share some molar processes in common, though some of the subprocesses differ. The ODR task requires sustained attention, so that the subject encodes the location of the target to be held on line over the delay. In contrast, the antisaccade task requires a covert shift of exogenous attention from the central fixation to the peripheral cue. Furthermore, the antisaccade task requires a spatial transformation from the position of the cue to the position of the saccade goal –-two positions which are usually in the mirror opposite locations. Both tasks, however, require inhibition. The finding that bipolar patients are able to perform ODR tasks as well as healthy control subjects suggests that their antisaccade task impairments may not be due to an inability to inhibit responses, but rather to a difficulty inhibiting responses when it is appropriate to do so, i.e., difficulty implementing inhibition. This interpretation of bipolar patients’ antisaccade task deficits is consistent with their antisaccade task deficits being state-dependent as well. This could also account for other individuals with mood disorders and/or affective lability; perhaps due to their mood disturbances, they are more susceptible to and/or more easily distracted by environmental stimuli such as the peripheral cue and thus are unable to implement inhibition in a context in which another response is more potent.
Table 1.
Component Processes Implicated in Saccadic Task Paradigms
| PROCESS | Oculomotor Delayed Response Task | Antisaccade Task |
|---|---|---|
| Attention | Sustain attention | Covert shift of exogenous attention |
| Inhibition | Inhibit saccade during delay period | Inhibit prepotent reflexive saccade |
| Response Generation | Make a voluntary saccade | Make a voluntary saccade |
| Working Memory | Hold task goal on line to guide behavior | Hold task goal on line to guide behavior |
| Spatial Perception | Calculate spatial information (i.e. location) | Calculate spatial information (i.e., direction, location) |
One can also look at the patients’ performance across various saccadic tasks in order to discern more about the brain circuitry that is likely to be implicated in their underlying pathophysiology. Overall, all patient groups are likely to have normal saccadic eye movement brainstem circuits. Patients with Tourette syndrome (TS), attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), affective disorder, and schizophrenia have no obvious abnormalities of saccadic eye movement characteristics, provided the tasks are simple and the target of the saccade remains illuminated. Some reports indicate that these patient groups show altered latencies of visually-guided saccades. However, changes in latency of saccades are not generally considered a sign of brainstem alterations. Rather changes in latency are likely to reflect alterations in the descending drive to brainstem circuits (reviewed in Munoz and Everling, 2004).
Schizophrenia, ADHD, and TS patient groups show deficits that are consistent with the overarching hypothesis that they are unable to make eye movements to goals that are represented internally. Striking saccade abnormalities appear in these adult psychiatric patients in tasks that rely on memory, prediction or other higher-order cognitive processing. For example, one common theme that appears in this complex and at times contradictory literature is that during performance of the antisaccade task, patients with schizophrenia, ADHD and TS all have increased error rates. Most of the studies agree that each of these patient groups has an inability to inhibit the reflexive prosaccade to the cue. Thus, one general conclusion is in all three patient groups, there is disruption in the circuitry underlying suppression of unwanted responses, i.e., the frontostriatal circuitry. Indeed, these patients’ inhibitory deficits in the antisaccade task suggest a dysfunction in the top-down inhibition of saccade brainstem circuits, largely consistent with a deficit in the DLPFC (see glossary).
Patients with DLPFC lesions show increased prosaccade errors in the antisaccade task (Fukushima, Fukushima, Miyasaka, & Yamashita, 1994; Guitton, Buchtel, & Douglas, 1985; Gooding et al., 1997; Pierrot-Deseilligny et al., 1991; Machado & Rafal, 2004). In the Fukushima et al. (1994) investigation, the frontal lesion patients displayed higher error rates as well as longer latencies in the antisaccade task compared to the control subjects. Pierrot-Deseilligny et al. (1991) conducted a comparative study of a group of patients with neurological lesions located in various frontal (FEF, supplementary motor area, DLPFC) regions with subjects with posterior parietal lesions and normal controls. They observed that only those patients with dorsolateral prefrontal lesions had a significantly higher error rate than the controls on the antisaccade task.
In patients with schizophrenia, ADHD and TS there are variable reports of increases in saccade latency. Thus, those patients with latency changes in addition to changes in error rate on the antisaccade task may point toward involvement of multiple cortical areas in their psychiatric illness. The differences in the magnitude and number of additional saccadic eye movement abnormalities relative to healthy controls may suggest the relative extent of dysfunction in the corticostriatal circuitry. For example, the psychiatric patient groups also differ in terms of their ability to perform tasks requiring sequencing of saccadic eye movements. Schizophrenia patients make fewer exploratory eye movements on visual search tasks than depressive patients and healthy controls. This finding supports the assertion that schizophrenia patients’ frontal dysfunction is more extensive than that found in the other psychiatric groups compared here, with additional disruption in the SEF, which are involved in cortical control of internally guided decision-making and sequencing of saccades (Tehovnik, Sommer, Chou, Slocum, & Schiller, 2000; Gold & Shadlen, 2007). We also noted that depressive patients with the melancholic subtype of the disorder experience significant difficulty on a task requiring reprogramming of saccadic eye movements. This finding suggests that the melancholic subtype of major depressive disorder may be more associated with corticostriatal dysfunction than other forms of depression (Rogers et al., 2000). Comparing patients with basal ganglia disease such as Parkinson’s Disease to those with psychiatric disease may shed further light on the neural substrates of deficits. In contrast to what one might predict, patients with Parkinson’s Disease often have reduced saccade latencies for prosaccades and often make more express saccades in the gap task than healthy, age-matched controls (Chan et al 2005). Interestingly although variably reported, Parkinson’s patients often make more prosaccade errors on the antisaccade task than healthy controls (Briand et al 1999; Fukushima et al., 1994; Vidailhet et al., 1994; Chan et al., 2005). Thus Parkinson’s Disease patients display an inability to suppress reflexive saccades as well as alterations in saccade latency. Deficits in the generation of both prosaccades and antisaccades suggests that these impairments may result from a similar underlying process that is altered in Parkinson’s Disease, whereas in schizophrenia, ADHD, TS and OCD, where prosaccades are largely intact and antisaccades are impaired, only part of the saccadic system is compromised.
Two recent investigations in nonhuman primates by Basso and Liu (Basso & Liu, 2007; Liu & Basso, 2008) demonstrate the effects of altering basal ganglia input on saccades. Electrical stimulation of an output nucleus of the basal ganglia resulted in a reduced latency and decreased variability in the latency of visually- guided saccades (Liu & Basso, 2008). The stimulation also resulted in an increased latency of memory guided saccades (Basso and Liu, 2007). Furthermore, the investigators observed that directional errors occurred only for memory saccades, whereas the direction of visually guided saccades was largely unaffected. It is noteworthy that basal ganglia dysfunction is implicated in both Parkinson’s Disease and schizophrenia. These phenomena are similar to the pattern of deficits generally observed in patients with Parkinson’s Disease (Briand, Strallow, Hening, Poizner, & Sereno, 1999; Brown et al., 1993; Corin, Elizan, & Bender, 1972; DeJong & Melvill Jones, 1971; Fukushima, Fukushima, Miyasaka & Yamashita, 1994) and schizophrenia. Through basal ganglia inputs to the superior colliculus (see glossary) (Liu and Basso, 2008) the resulting effects on saccades may appear very similar in spite of very different neuropathological processes. One hypothesis is that the alterations in dopaminergic tone that occur in either Parkinson’s Disease or schizophrenia may alter the signaling within the basal ganglia. Therefore, one interesting future direction would be to compare the variability in saccade reaction times across psychiatric patient populations. When prosaccades and antisaccades occur in rapid alternation, Tourette Syndrome patients are better than healthy controls subjects at suppressing the occurrence of reflexive saccades in the antisaccade task. This result argues strongly that TS patients are able to suppress movements even better than control subjects. Given that many TS patients practice suppressing their tics, this finding may be unique to this patient population. Nevertheless, the result may have occurred because of a compensatory mechanism in which subjects exert extra effort to suppress movements. Indeed, it is important to consider the possibility that compensatory strategies may influence saccade behavior whenever interpreting the results of oculomotor assays.
As this review illustrates, the study of saccadic eye movements holds great potential for the fields of psychiatry and neurology. Our survey of the extant literature also indicates considerable variability in saccadic performance both within and across patient populations. It may instructive to administer multiple permutations of the same task in the same study. This strategy can be used in order to create a more specific profile of the patient group’s behavioral performance across tasks, potentially providing more insight into the nature of the underlying dysfunction (Levy et al., 1998; Nieuwenhuis et al., 2004). Just as some investigators (Winograd-Gurvich et al., 2006a, 2006b) observed performance differences between depressive patients with versus without melancholia, it would be useful in future studies to parametrically alter saccadic tasks specifically to tease apart the mechanisms underlying the melancholic patients’ saccadic deficits. Furthermore, it is possible that in some patient groups, such as schizophrenia patients, different subtypes may have different mechanisms underlying their antisaccade deficits. Indeed, this area of inquiry would be advanced by including tasks in which the ability to inhibit reflexes in other modalities or across different contexts is assessed.
The variability in saccadic performance across patient populations could be used to clinical advantage. A potential clinical application of saccadic research would be the development of typical performance profiles across a battery of saccadic tasks for different psychiatric patient groups. In order to achieve this goal, researchers in this area would need to include multiple saccadic tasks in each battery on a regular basis. Once a sufficient database is developed, individuals’ performance on the saccadic battery could be compared against the prototypical profiles for each patient group. Sufficiently large samples would be needed to develop reliable profiles and to insure adequate statistical power. Research based on extremely large samples such as the COGS study (Radant et al., 2007) looks promising, though the researchers will be limited in the number of antisaccade task conditions they can include in their battery. This strategy namely, developing prototypical performance profiles based upon a combination of saccadic tasks and the use of several saccadic parameters, could be used to aid in the differential diagnosis of commonly misdiagnosed disorders, such as schizophrenia versus bipolar disorder, or progressive supranuclear palsy versus Parkinson’s Disease (see, for example, Antoniades, Bak, Carpenter, Hodges, & Barker, 2007).
Saccadic research also holds great promise for informing current efforts in the field of genetics. Genetic heterogeneity undoubtedly stymies progress in the effort to uncover the etiology of complex genetic disorders. Observations of performance differences between subgroups within a given diagnostic group can be used to help establish more homogeneous subtypes, perhaps improving the efficiency of gene detection. One example of a disorder in which this strategy would be helpful is Tourette’s Syndrome, which is sometimes accompanied by obsessive compulsive behavior and/or symptoms (Robertson, 2000). It would be interesting and informative to determine whether there are performance differences between Tourette’s patients with and without OCD. If there are reliable performance differences, these findings may help to assist subtyping before the genetic studies commence.
Another application for saccadic research lies in the area of pharmacology. With the exception of schizophrenia, in which antisaccade task performance appears to be a trait-related characteristic, saccadic task performance may be used to test the effects of pharmacological interventions. Test-retest designs investigating aspects of saccadic performance can be used to determine the efficacy of pharmacological agents purported to improve attentional functioning and/or motor control. Alternatively, clinicians could refer patients to investigators for oculomotor assays in order to test for adverse effects of medications on cognitive functions such as attention, memory, and reaction time. In summary, combining batteries of saccade tasks with state of the art genetic, pharmacological and clinical tools/procedures holds much promise for unlocking the mysteries of heterogeneous and varied psychiatric illness.
Abbreviations
- ACC
anterior cingulate cortex
- ADHD
attention deficit hyperactivity disorder
- BOLD
blood oxygen level dependent (used in fMRI)
- CPT
Continuous Performance Test
- DLPFC
dorsolateral prefrontal cortex
- DSM
Diagnostic and Statistical Manual
- DTI
diffusion tensor imaging
- ERPs
Event Related Potentials
- FEF
frontal eye fields
- fMRI
functional Magnetic Resonance Imaging
- OCD
obsessive compulsive disorder
- PANSS
Positive and Negative Symptom Scale
- PD
Parkinson’s Disease
- SEF
supplementary eye fields
- Trails A
Trail Making Test Part A
- Trails B
Trail Making Test Part B
- TS
Tourette’s Syndrome
- WCST
Wisconsin Card Sorting Test
Footnotes
Much of our understanding of the oculomotor system comes from our study of nonhuman primates. Readers are referred to Johnson and Everling’s (2008) review of basic research regarding the neural circuitry controlling eye movements, also provided in this special volume.
Although some researchers (Cohen & Servan-Schreiber, 1992; Goldman-Rakic, 1987; Kimberg & Farah, 1993; Kimberg D’Esposito, & Farah, 1997) view inhibition as being subsumed under working memory, others (c.f. Fuster, 1985, 1997; Roberts et al., 1994) conceptualize the two processes as discrete but interrelated functions. For the purpose of this review, we have chosen to treat working memory and inhibition as separate processes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends in Neurosciences. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Allen JS, Lambert AJ, Johnson FY, Schmidt K, Nero KL. Antisaccadic eye movements and attentional asymmetry in schizophrenia in three Pacific populations. Acta Psychiatrica Scandinavica. 1996;94:258–265. doi: 10.1111/j.1600-0447.1996.tb09858.x. [DOI] [PubMed] [Google Scholar]
- Amado I, Landgraf S, Bourdel M-C, Leonardi S, Krebs M-O. Predictive saccades are impaired in biological nonpsychotic siblings of schizophrenia patients. Journal of Psychiatry and Neuroscience. 2008;33:17–22. [PMC free article] [PubMed] [Google Scholar]
- Antoniades CA, Bak TH, Carpenter RHS, Hodges JR, Barker RA. Diagnostic potential of saccadometry in progressive supranuclear palsy. Biomarkers Medicine. 2007;1:487–490. doi: 10.2217/17520363.1.4.487. [DOI] [PubMed] [Google Scholar]
- Bagary MS, Hutton SB, Symms MR, Barker GJ, Mutsatsa SH, Barnes TRE, Joyce EM, Ron MA. Structural neural networks subserving oculomotor function in first-episode schizophrenia. Biological Psychiatry. 2004;56:620–627. doi: 10.1016/j.biopsych.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Bahill AT, Clark M, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Biosciences. 1975;24:191–204. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, va IJenzdoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova MV, Lindgren K, Goff DC, Intriligator JM, Manoach DS. Antisaccades and task switch. Studies of control processes in saccadic function in normal subjects and schizophrenic patients. Annals of the New York Academy of Science. 2002;956:250–263. doi: 10.1111/j.1749-6632.2002.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova MV, Linden KA, Goff DC, Manoach DS. What is perseverated in schizophrenia? Evidence of abnormal response plasticity in the saccadic system. Journal of Abnormal Psychology. 2005;114:75–84. doi: 10.1037/0021-843X.114.1.75. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Pandita M, Thakkar K, Goff DC, Manoach DS. The relation between antisaccade errors, fixation stability and prosaccade errors in schizophrenia. Experimental Brain Research. 2008;186:273–282. doi: 10.1007/s00221-007-1235-2. [DOI] [PubMed] [Google Scholar]
- Basso MA, Liu P. Context-dependent effects of Substantia Nigra stimulation on eye movements. Journal of Neurophysiology. 2007;97:4129–4142. doi: 10.1152/jn.00094.2007. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Lenzenweger MF, Waller N. Schizotypy, Taxometrics, and Disconfirming Theories in Soft Science. Comment on Rawlings, Williams, Haslam, and Claridge. Individual and Personality Differences. 2008 in press. [Google Scholar]
- Becker W. Metrics. In: Wurtz RH, Goldberg ME, editors. The Neurobiology of Saccadic Eye Movements. Elsevier; Amsterdam: 1989. pp. 13–67. [PubMed] [Google Scholar]
- Boudet C, Bocca ML, Chabot B, Delamillieure P, Brazo P, Denise P, Dollfus S. Are eye movement abnormalities indicators of genetic vulnerability to schizophrenia? European Psychiatry. 2005;30:339–345. doi: 10.1016/j.eurpsy.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Burke JG, Reveley MA. Improved antisaccade performance with risperidone in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:449–454. doi: 10.1136/jnnp.72.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, McDowell JE, Cadenhead KS, Clementz BA. Saccadic inhibition among schizotypal personality disorder subjects. Psychophysiology. 2001;38:399–403. [PubMed] [Google Scholar]
- Brendel GR, Stern E, Silbersweig DA. Defining the neurocircuitry of borderline personality disorder: Functional neuroimaging approaches. Development and Psychopathology. 2005;17:1197–1206. doi: 10.1017/s095457940505056x. [DOI] [PubMed] [Google Scholar]
- Briand KA, Strallow D, Hening W, Poizner H, Sereno AB. Control of voluntary and reflexive saccades in Parkinson’s disease. Experimental Brain Research. 1999;129:38–48. doi: 10.1007/s002210050934. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Kennard C. Predictive eye saccades are different from visually triggered saccades. Vision Research. 1987;27:517–520. doi: 10.1016/0042-6989(87)90037-x. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Schwarz U, Bowman EM, Fuhr P, Robinson DL, Hallett M. Dopamine dependent reaction time deficits in patients with Parkinson’s disease are task specific. Neuropsychologia. 1993;31:459–469. doi: 10.1016/0028-3932(93)90060-d. [DOI] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biological Psychiatry. 2006;60:235–241. doi: 10.1016/j.biopsych.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Cegalis JA, Sweeney JA, Dellis EM. Refixation saccades and attention in schizophrenia. Psychiatry Research. 1982;7:189–198. doi: 10.1016/0165-1781(92)90092-h. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia. 2005;43:784–96. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin Body-image aberration in schizophrenia. Journal of Abnormal Psychology. 1983;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Clementz BA. The ability to produce express saccades as a function of gap interval among schizophrenia patients. Experimental Brain Research. 1996;111:121–130. doi: 10.1007/BF00229561. [DOI] [PubMed] [Google Scholar]
- Clementz B, McDowell J, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. Journal of Abnormal Psychology. 1994;103:277–287. [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. A neural network model of disturbances in the processing of context in schizophrenia. Psychiatric Annals. 1992;22:131–236. [Google Scholar]
- Corin MS, Elizan TS, Bender MB. Oculomotor function in patients with Parkinson’s disease. Journal of the Neurological Sciences. 1972;15:251–265. doi: 10.1016/0022-510x(72)90068-8. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. American Journal of Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Iacono WG. Saccadic disinhibition in schizophrenia patients and their first-degree biological relatives: a parametric study of the effects of increasing inhibitory load. Experimental Brain Research. 2001;137:228–236. doi: 10.1007/s002210000635. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L. Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychological Medicine. 1995;25:461–471. doi: 10.1017/s0033291700033389. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Puri BK, Nijran KS, Jones B, Kennard C, Lewis SW. Abnormal saccadic distractibility in patients with schizophrenia: A 99mTc-HMPA) SPET study. Psychological Medicine. 1996;26:265–277. doi: 10.1017/s0033291700034668. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW. Saccadic eye movements in families multiply affected with schizophrenia: The Maudsley Family Study. American Journal of Psychiatry. 1998;155:1703–1710. doi: 10.1176/ajp.155.12.1703. [DOI] [PubMed] [Google Scholar]
- Crevits L, Van den Abbeele D, Audenaert K, Goethals M, Dierick M. Effect of repetitive transcranial magnetic stimulation on saccades in depression: A pilot study. Psychiatry Research. 2005;135:113–119. doi: 10.1016/j.psychres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- DeJong JD, Melvill Jones G. Akinesia, hypokinesia, and bradykinesia in the oculomotor system of patients with Parkinson’s disease. Experimental Neurolology. 1971;32:58–68. doi: 10.1016/0014-4886(71)90165-8. [DOI] [PubMed] [Google Scholar]
- Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayll JX, Kin NNY, Lai S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- De Renzi E. Oculomotor disturbances in hemispheric disease. In: Johnston CW, Pirozzolo FJ, editors. Neuropsychology of Eye Movements. Hillsdale, NJ: Erlbaum; 1988. pp. 177–195. [Google Scholar]
- deWilde OM, Dingemans P, Boeree T, Linszen D. Antisaccade deficit is present in young first-episode patients with schizophrenia but not in their healthy young siblings. Psychological Medicine. 2008;38:871–875. doi: 10.1017/S0033291707001894. [DOI] [PubMed] [Google Scholar]
- Diefendorf AR, Dodge R. An experimental study of ocular reaction of the insane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- Done DJ, Frith CD. Automatic and strategic control of eye movements in schizophrenia. In: Gale AG, Johnson F, editors. Theoretical and Applied Aspects of Eye Movement Research. North-Holland: Elsevier; 1984. pp. 481–487. [Google Scholar]
- Donohoe G, Reilly R, Clarke S, Meredith S, Green B, Morris D, Gil M, Corvin Ai, Gravan Robertson IA. Do antisaccade deficits in schizophrenia provide evidence of a specific inhibitory function? Journal of the International Neuropsychological Society. 2006;12:901–906. doi: 10.1017/S135561770606108X. [DOI] [PubMed] [Google Scholar]
- Duncan J. Attention, intelligence, and the frontal lobes. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 721–733. [Google Scholar]
- Dursun SM, Burke JG, Reveley MA. Antisaccade eye movement abnormalities in Tourette syndrome: evidence for cortico-striatal network dysfunction? Journal of Psychopharmacology. 2000;14:37–39. doi: 10.1177/026988110001400104. [DOI] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51:215–222. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Engelken EJ, Stevens KW, Enderle JD. Computer analysis of smooth pursuit eye movements. Biomedical Sciences Instrumentation. 1989;25:127–133. [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Chitnis XA, Corr PJ, Crawford TJ, Fannon DG, O’Ceallaigh SO, Sumich AL, Doku VC, Sharma T. Volumetric neural correlates of antisaccade eye movements in first-episode psychosis. American Journal of Psychiatry. 2004a;161:1918–1921. doi: 10.1176/ajp.161.10.1918. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Corr PJ, Das M, Zachariah E, Hughes C, Sumich AL, Rabe-Hesketh S, Sharma T. Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. Journal of Psychiatric Research. 2004b;38:177–184. doi: 10.1016/s0022-3956(03)00105-5. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari, Crawford TJ, Davis RE, Sharma T, Corr PJ. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology. 2003;40:620–628. doi: 10.1111/1469-8986.00063. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Flak V, Sharma T, Davis RE, Corr PJ. Saccadic eye movements, schizotypy, and the role of neuroticism. Biological Psychology. 2005;68:61–78. doi: 10.1016/j.biopsycho.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Hall M-H, Schulze K, Toulopoulo T, Landau S, Crawford TJ, Murray RM. Antisaccade performance in monozygotic twins discordant for schizophrenia: The Maudsley Twin Study. American Journal of Psychiatry. 2006;163:543–545. doi: 10.1176/appi.ajp.163.3.543. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Preuss S, Brand A, Flohr H. Hypometric primary saccades of schizophrenics in a delayed response task. Experimental Brain Research. 1996;111:289–296. doi: 10.1007/BF00227306. [DOI] [PubMed] [Google Scholar]
- Farber RH, Swerdlow NR, Clementz BA. Saccadic performance characteristics and the behavioral neurology of Tourette’s syndrome. Journal of Neurology, Neurosurgery and Psychiatry. 1999;66:305–312. doi: 10.1136/jnnp.66.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiefel D, Farber RH, Clementz BA, Perry W, Anilo-Vento L. Inhibitory deficits in ocular motor behavior in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2004;56:333–339. doi: 10.1016/j.biopsych.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: Extremely short reaction times of goal directed eye movements. Experimental Brain Research. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Fisher B, Weber H. Express saccades and visual attention. Behavioral Brain Sciences. 1993;16:553–610. [Google Scholar]
- Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biological Psychiatry. 1988;23:670–677. doi: 10.1016/0006-3223(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Miyasaka K, Yamashita I. Voluntary control of saccadic eye movement in patients with frontal cortical lesions and parkinsonian patients in comparison with that in schizophrenics. Biological Psychiatry. 1994;36:21–30. doi: 10.1016/0006-3223(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Morita N, Yamashita I. Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biological Psychiatry. 1990;28:943–958. doi: 10.1016/0006-3223(90)90060-f. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex and temporal integration. In: Jones EG, Peters A, editors. Cerebral cortex: Vol. 4. Association and auditory cortices. New York: Plenum; 1985. pp. 151–177. [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neurpspychology of the frontal lobe. 3. New York: Plenum; 1997. [Google Scholar]
- Frith CD. The cognitive neuropsychology of schizophrenia. Lawrence Erlbaum; East Sussex, UK: 1992. [Google Scholar]
- Gaebel W, Ulrich G, Frick K. Visuomotor performance of schizophrenic patients and normal controls in a picture viewing task. Biological Psychiatry. 1987;22:1227–1237. doi: 10.1016/0006-3223(87)90030-8. [DOI] [PubMed] [Google Scholar]
- Gold JL, Shadlen MN. The neural basis of decision making. Annual Review of Neuroscience. 2007;20:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Plum F, Mountcastle V, editors. Handbook of Physiology. Vol. 5. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS. Working memory in schizophrenia. Journal of Neuropsychiatry. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gooding DC. Antisaccade task performance in questionnaire-identified schizotypes. Schizophrenia Research. 1999;35:157–166. doi: 10.1016/s0920-9964(98)00120-0. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Grabowski JA, Hendershot CS. Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Research. 2000;97:119–112. doi: 10.1016/s0165-1781(00)00226-2. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Iacono WG. Schizophrenia through the lens of a developmental psychopathology perspective. In: Cicchetti D, Cohen DJ, editors. Manual of Developmental Psychopathology. Risk, disorder, and adaptation. II. New York: Wiley; 1995. pp. 535–580. [Google Scholar]
- Gooding DC, Iacono WG, Grove WM. Ocular motor performance in schizophrenic patients and neurological patients. Schizophrenia Research. 1997;24:242–243. [Google Scholar]
- Gooding DC, Iacono WG, Hanson DR. Smooth pursuit and saccadic eye movement performance in a prefrontal leukotomy patient. Journal of Psychiatry & Neuroscience. 1999;24:462–467. [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Mohapatra L, Shea HB. Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychological Medicine. 2004;34:921–932. doi: 10.1017/s003329170300165x. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Shea HB, Matts CW. Saccadic performance in questionnaire-identified schizotypes over time. Psychiatry Research. 2005;133:173–186. doi: 10.1016/j.psychres.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. Journal of Nervous and Mental Disease. 2001;189:8–16. doi: 10.1097/00005053-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Grootens KP, van Luijtelaar G, Buitelaar JK, van der Laan A, Hummelen JW, Verkes RJ. Inhibition errors in borderline personality disorder with psychotic-like symptoms. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008;32:267–273. doi: 10.1016/j.pnpbp.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Gouras P. Oculomotor system. In: Kandel E, Schwartz JH, editors. Principles of Neural Science. 2. London: Elsevier; 1985. pp. 571–583. [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Experimental Brain Research. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hallet PE. Primary and secondary saccades to goals defined by instruction. Vision Research. 1987;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hallet PE, Adams The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Research. 1980;20:329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Harris MSH, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naïve first-episode schizophrenia. Psychological Medicine. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. Journal of Neurophysiology. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Holahan A-LV, O’Driscoll GA. Antisaccade and smooth pursuit performance in positive- and negative-symptom schizotypy. Schizophrenia Research. 2005;76:43–54. doi: 10.1016/j.schres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Clem T, Litman R, Pickar D. Maladaptive anticipatory saccades in schizophrenia. Biological Psychiatry. 1991;30:779–794. doi: 10.1016/0006-3223(91)90234-d. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: A visual scanpath study of emotional expression processing. Anxiety Disorders. 2003;17:33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Hutton SB. Cognitive processes involved in reflexive and volitional saccades. Brain and Cognition. 2008 in press. [Google Scholar]
- Hutton SB. Improved antisaccade performance in schizophrenia with risperidone. Commentary. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:429. doi: 10.1136/jnnp.72.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SB, Crawford TJ, Puri BK, Duncan L-J, Chapman M, Kennard C, Barnes TRE, Joyce EM. Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychological Medicine. 1998;28:685–692. doi: 10.1017/s0033291798006722. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Joyce EM, Barnes TR, Kennard C. Saccadic distractibility in first episode schizophrenia. Neuropsychologia. 2002;40:1729–1736. doi: 10.1016/s0028-3932(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Tuason VB, Johnson RA. Dissociation of smooth-pursuit and saccadic eye tracking in remitted schizophrenics. Archives of General Psychiatry. 1981;38:991–996. doi: 10.1001/archpsyc.1981.01780340043005. [DOI] [PubMed] [Google Scholar]
- Jackson G, Mueller S, Hambleton K, Hollis C. Enhanced cognitive control in Tourette Syndrome during task uncertainty. Experimental Brain Research. 2007;182:357–364. doi: 10.1007/s00221-007-0999-8. [DOI] [PubMed] [Google Scholar]
- Karoumi B, Ventre-Dominey J, Vighetto A, Dalery J, d’Amato Saccadic eye movements in schizophrenic patients. Psychiatry Research. 1998;77:9–19. doi: 10.1016/s0165-1781(97)00126-1. [DOI] [PubMed] [Google Scholar]
- Kennard C. Scanpaths: The path to understanding abnormal cognitive processing in neurological disease. Annals of the New York Academy of Sciences. 2002;956:242–249. doi: 10.1111/j.1749-6632.2002.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Cognitive functions in the prefrontal cortex: Working memory and executive control. Current Directions in Psychological Science. 1997;6:185–192. [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: The role of working memory in complex, organized behavior. Journal of Experimental Psychology: General. 1993;122:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Klein CH, Brugner G, forester F, Mller W, Schweickhardt A. The gap effect in pro-saccades and anti-saccades in psychometric schizotypes. Biological Psychology. 2000;55:25–39. doi: 10.1016/s0301-0511(00)00062-4. [DOI] [PubMed] [Google Scholar]
- Klein C, Heinks T, Andresen B, Berg P, Moritz S. Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biological Psychiatry. 2000;47:978–990. doi: 10.1016/s0006-3223(00)00234-1. [DOI] [PubMed] [Google Scholar]
- Klein C. Developmental functions for parameters derived from pro- and anti-saccade tasks in 199 participants aged 6–28 years. Experimental Brain Research. 2001;139:1–17. doi: 10.1007/s002210100711. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsushima E, Ando K, Ando H, Sakurada M, Ohta K, Moriya H, Shimazono Y. Exploratory eye movements and neuropsychological tests in schizophrenic patients. Schizophrenia Bulletin. 1992;18:85–94. doi: 10.1093/schbul/18.1.85. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsushima E, Nakajima K, Shiraishi H, Ando K, Ando H, Shimazono Y. Eye movements in acute, chronic, and remitted schizophrenics. Biological Psychiatry. 1990;27:975–989. doi: 10.1016/0006-3223(90)90035-z. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsushima E, Ohta K, Toru M, Han Y-H, Shen Y-C, Moussaoui D, David I, Sato K, Yamashita I, Kathmann N, Hippius H, Thavundayil JX, Lal S, Nair NPV, Potkin SG, Prilipko L. Stability of exploratory eye movements as a marker of schizophrenia: a WHO multi-center study. Schizophrenia Research. 2001;52:203–213. doi: 10.1016/s0920-9964(00)00181-x. [DOI] [PubMed] [Google Scholar]
- Kojima T, Potkin SG, Kharazmi M, Matsushima E, Herrera J, Shimazono Y. Limited eye movement patterns in chronic schizophrenic patients. Psychiatry Research. 1989;28:307–314. doi: 10.1016/0165-1781(89)90211-4. [DOI] [PubMed] [Google Scholar]
- Kumari V, Ettinger U, Crawford TJ, Zachhariah E, Sharama T. Lack of association between prepulse inhibition and antisaccadic deficits in chronic schizophrenia: Implications for identification of schizophrenia endophenotypes. Journal of Psychiatric Research. 2005;39:227–240. doi: 10.1016/j.jpsychires.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Krebs M-O, Gut-Fayand A, Amado I, Dabn C, Bourdel M-C, Poirier M-F, Berthoz A. Impairment of predictive saccades in schizophrenia. NeuroReport. 2001;12:465–469. doi: 10.1097/00001756-200103050-00009. [DOI] [PubMed] [Google Scholar]
- Landgraf S, Amado I, Bourdel M-C, Leonardi S, Krebs M-O. Memory-guided saccade abnormalities in schizophrenic patients and their healthy, full biological siblings. Psychological Medicine. 2008;38:861–870. doi: 10.1017/S0033291707001912. [DOI] [PubMed] [Google Scholar]
- Larrison AL, Ferrante CF, Briand KA, Sereno AB. Schizotypal traits, attention, and eye movements. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2000;24:357–372. doi: 10.1016/s0278-5846(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Larrison-Faucher AL, Matorin AA, Sereno AB. Nicotine reduces antisaccade errors in task impaired schizophrenic subjects. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2004;28:505–516. doi: 10.1016/j.pnpbp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington’s disease: Initiation defects and distractibility. Neurology. 1987;37:364–370. doi: 10.1212/wnl.37.3.364. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. Philadelphia: Davis Co; 1983. [Google Scholar]
- LeVasseur AL, Flanagan JR, Riopelle RJ, Munoz DP. Control of volitional and reflexive saccades in Tourette’s syndrome. Brain. 2001;124:2045–2058. doi: 10.1093/brain/124.10.2045. [DOI] [PubMed] [Google Scholar]
- Levin S, Holzman PS, Rothenberg SJ, Lipton RB. Saccadic eye movements in psychotic patients. Psychiatry Research. 1981;5:47–58. doi: 10.1016/0165-1781(81)90060-3. [DOI] [PubMed] [Google Scholar]
- Levin S, Jones A, Stark L, Merrin EL, Holzman PS. Saccadic eye movements of schizophrenic patients measured by reflected light technique. Biological Psychiatry. 1982;17:1277–1287. [PubMed] [Google Scholar]
- Levy DL, Mendell NR, LaVancher GA, Brownstein J, Krastoshevsky O, Teraspulsky L, McManus KS, Lo Y, Bloom R, Matthysse S, Holzman PS. Disinhibition in antisaccade performance in schizophrenia. In: Lenzenweger MF, Dworkin R, editors. Origins and Development of Schizophrenia: Advances in Experimental Psychopathology. Washington, DC: American Psychological Association Press; 1998. pp. 185–210. [Google Scholar]
- Lezak M. Neuropsychological assessment. 2. New York: Oxford University Press; 1983. [Google Scholar]
- Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. Journal of Neurophysiology. 2008 doi: 10.1152/jn.01043.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louchart-de la Chapelle S, Nkam I, Houy E, Belmont A, Menard J-F, Roussignol A-C, Siwek O, Mezerai M, Guillermou M, Fouldrin G, Levillain D, Dollfus S, Campion D, Thibaut F. A concordance study of three electrophysiological measures in schizophrenia. American Journal of Psychiatry. 2005;162:466–474. doi: 10.1176/appi.ajp.162.3.466. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Simon H, Zanelli JW, Walwyn R, McDonald CD, Murray RM. Saccadic distractibility is elevated in schizophrenia patients, but not in their unaffected relatives. Psychological Medicine. 2005;35:1727–1736. doi: 10.1017/S0033291705005738. [DOI] [PubMed] [Google Scholar]
- Machado L, Rafal RD. Control of fixation and saccades during an anti-saccade task: an investigation in humans with chronic lesions of oculomotor cortex. Experimental Brain Research. 2004;156:55–63. doi: 10.1007/s00221-003-1765-1. [DOI] [PubMed] [Google Scholar]
- Mackert A, Flechtner M. Saccadic reaction times in acute and remitted schizophrenics. European Archives of Psychiatry and Neurological Sciences. 1989;239:33–38. doi: 10.1007/BF01739741. [DOI] [PubMed] [Google Scholar]
- Mahlberg R, Steinacher B, Mackert A, Flechtner K-M. Basic parameters of saccadic eye movements: Differences between unmedicated schizophrenia and affective disorder patients. European Archives of Psychiatry and Clinical Neurosciences. 2001;251:205–210. doi: 10.1007/s004060170028. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Ketwaroo GA, Poli FE, Thakkar KN, Barton JJS, Goff DC, Fischl B, Vangel M, Tuch DS. Reduced microstructural integrity of the white matter underlying anterior cingulated cortex is associated with increased saccadic latency in schizophrenia. NeuroImage. 2007;37:599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Maruff PJ, Danckert C, Pantellis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychological Medicine. 1998;28:1091–1100. doi: 10.1017/s0033291798007132. [DOI] [PubMed] [Google Scholar]
- Maruff P, Pantelis C, Danckert J, Smith D, Currie J. Deficits in the endogenous redirection of covert visual attention in chronic schizophrenia. Neuropsychologia. 1996;34:1079–1084. doi: 10.1016/0028-3932(96)00035-8. [DOI] [PubMed] [Google Scholar]
- Maruff P, Purcell R, Tyler P, Pantelis C, Currie J. Abnormalities of internally generated saccades in obsessive-compulsive disorder. Psychological Medicine. 1999;29:1377–1385. doi: 10.1017/s0033291799008843. [DOI] [PubMed] [Google Scholar]
- Martin LF, Hall M-H, Ross RG, Zerbe G, Freedman R, Olincy A. Physiology of schizophrenia, bipolar disorder, and schizoaffective disorder. American Journal of Psychiatry. 2007;164:1900–1906. doi: 10.1176/appi.ajp.2007.06010017. [DOI] [PubMed] [Google Scholar]
- Mather JA, Putchat C. Motor control of schizophrenics--I. Oculomotor control of schizophrenics: A deficit in sensory processing, not strictly in motor control. Journal of Psychiatric Research. 1983;17:343–360. doi: 10.1016/0022-3956(82)90040-1. [DOI] [PubMed] [Google Scholar]
- Matsushima E, Kojima T, Ohta K, Obayashi S, Nakajima K, Kakuma T, Ando H, Ando K, Toru M. Exploratory eye movement dysfunctions in patients with schizophrenia: Possibility as a discriminator for schizophrenia. Journal of Psychiatric Research. 1998;32:289–295. doi: 10.1016/S0022-3956(98)00019-3. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biological Psychiatry. 2002;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. Ocular motor delayed-response task performance among schizophrenia patients. Neuropsychobiology. 1996;34:67–71. doi: 10.1159/000119294. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Experimental Brain Research. 1997;115:333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. Behavioral and brain imaging studies of saccadic performance in schizophrenia. Biological Psychology. 2001;57:5–22. doi: 10.1016/s0301-0511(01)00087-4. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA, Wixted JT. Timing and amplitude during predictive saccadic tracking in schizophrenia. Psychophysiology. 1996;33:93–101. doi: 10.1111/j.1469-8986.1996.tb02112.x. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin B, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain and Cognition. 2008 doi: 10.1016/j.bandc.2008.08.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz B. Ocular motor delayed response task performance among schizophrenia patients and their biological relatives. Psychophysiology. 2001;38:153–156. [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Moser A, Kompf D, Arolt V, Resch T. Quantitative analysis of eye movements in schizophrenia. Neuro-Opthalmology. 1990;10:73–80. [Google Scholar]
- Muller N, Riedel M, Eggert T, Straube A. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients. Part II. Saccadic latency, gain, and fixation suppression errors. European Archives of Psychiatry and Clinical Neuroscience. 1999;249:7–14. doi: 10.1007/s004060050059. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. Enhanced cognitive control in young people with Tourette’s Syndrome. Current Biology. 2006;16:570–573. doi: 10.1016/j.cub.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in Attention-Deficit Hyperactivity Disorder. Journal of Neurophysiology. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nature Neuroscience. 2004;5:218–227. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Kojima T, Matsushima E, Uesugi S, Ando K, Ohtaka T, Takahashi R, Ohnuma T, Shimazono Y. Eye movements in patients with temporal lobe epilepsy--comparison with schizophrenics and normal controls. The Japanese Journal of Psychiatry and Neurology. 1988;42:632–634. doi: 10.1111/j.1440-1819.1988.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff SE, Radant AD, Reichler R, Hommer DW. Saccadic eye movements and soft signs in Obsessive-Compulsive Disorder. Psychiatry Research. 1991;38:173–185. doi: 10.1016/0165-1781(91)90042-n. [DOI] [PubMed] [Google Scholar]
- Nieman D, Becker H, van de Fliert R, Plat N, Bour L, Koelman H, Klaassen M, Dingemans P, Niessen M, Linszenn D. Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophrenia Research. 2007;95:54–60. doi: 10.1016/j.schres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Broerse A, Nielen MMA, deJong R. A goal activation approach to the study of executive function: An application to antisaccade tasks. Brain and Cognition. 2004;56:198–214. doi: 10.1016/j.bandc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Nkam I, Thibaut F, Denise P, Van Ser Elst A, Segard L, Brazo P, Menard J-F, Thery S, Halbeck I, Delamilleure P, Vasse T, Etard O, Dollfus S, Champion D, Levillain D, Petit M. Saccadic and smooth-pursuit eye movements in deficit and non-deficit schizophrenia. Schizophrenia Research. 2001;48:145–153. doi: 10.1016/s0920-9964(99)00165-6. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proceedings of the National of Sciences. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll GA, Lenzenweger MF, Holzman PS. Antisaccades and smooth pursuit eye tracking and schizotypy. Archives of General Psychiatry. 1998;55:837–843. doi: 10.1001/archpsyc.55.9.837. [DOI] [PubMed] [Google Scholar]
- Park S. Association of an oculomotor delayed response task and the Wisconsin Card Sort Test in schizophrenic patients. International Journal of Psychophysiology. 1997;27:147–171. doi: 10.1016/s0167-8760(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Park SH, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park SH, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophrenia Research. 1993;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C. Saccade and smooth pursuit impairment after cerebral hemispheric lesions. European Neurology. 1994;34:121–134. doi: 10.1159/000117025. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJS, Thakkar KN, Greve DN, Goff DS, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulated circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Radant AD, Claypoole K, Wingerson DK, Cowley DS, Roy-Byrne PP. Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biological Psychiatry. 1997;42:797–805. doi: 10.1016/s0006-3223(96)00464-7. [DOI] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, et al. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophrenia Research. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, Van der Linden JA, Kahn RS, Ramsey NF. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Archives of General Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- Ramchandran RS, Manoach DS, Cherkasova MV, Lindgren KA, Goff DC, Barton JJS. The relationship of saccadic peak velocity to latency: Evidence for a new prosaccadic abnormality in schizophrenia. Experimental Brain Research. 2004;159:99–107. doi: 10.1007/s00221-004-1940-z. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MSH, Khine TT, Keshavan MS, Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biological Psychiatry. 2008;63:776–783. doi: 10.1016/j.biopsych.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop J, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain and Cognition. 2008 doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischies FM, Stieglitz R-D, Mielewczyk A, Vogel A. Impaired performance in a saccadic tracking task in schizophrenic patients. European Archives of Psychiatry and Neurological Sciences. 1989;239:58–61. doi: 10.1007/BF01739745. [DOI] [PubMed] [Google Scholar]
- Reuter B, Kathmann N. Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychologica. 2004;115:255–269. doi: 10.1016/j.actpsy.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Reuter B, Rakusan L, Kathmann N. Poor antisaccade performance in schizophrenia: An inhibition deficit? Psychiatry Research. 2005;135:1–10. doi: 10.1016/j.psychres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Hughes HC, Fendrich R. The reduction of saccadic latency by prior offset of the fixation point: An analysis of the gap effect. Perception and Psychophysics. 1991;49:167–175. doi: 10.3758/bf03205036. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Hager LD, Heron C. Prefrontal cognitive processes: Working memory and inhibition in the antisaccade task. Journal of Experimental Psychology: General. 1994;123:374–393. [Google Scholar]
- Robertson MM. Tourette syndrome, associated conditions, and the complexities of treatment. Brain. 2000;123:425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Bradshaw JL, Phillips JG, Chiu E, Vaddakdi K, Presnel I, Mileshkin C. Parkinsonian motor characteristics in Unipolar Major Depression. Journal of Clinical and Experimental Neuropsychology. 2000;22:232–244. doi: 10.1076/1380-3395(200004)22:2;1-1;FT232. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Dick EL, O’Hearn K, Sweeney JA. Response-inhibition deficits in obsessive-compulsive disorder: An indicator of dysfunction in frontostriatal circuits. Journal of Psychiatry and Neuroscience. 1997;22:29–38. [PMC free article] [PubMed] [Google Scholar]
- Ross DE, Ochs AL, Hill MR, Goldberg SC, Pandurangi AK, Winfrey CJ. Erratic eye tracking in schizophrenic patients as revealed by high resolution techniques. Biological Psychiatry. 1998;24:675–688. doi: 10.1016/0006-3223(88)90141-2. [DOI] [PubMed] [Google Scholar]
- Ross RG, Harris JG, Olincy A, Radant A. Eye movement task measures inhibition and spatial working memory in adults with schizophrenia, AHD, and a normal comparison group. Psychiatric Research. 2000;95:35–42. doi: 10.1016/s0165-1781(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Schwartz BL, Kim SJ, Deutsch SJ. Correlation between antisaccade and Wisconsin Card Sorting Test performances in schizophrenia. American Journal of Psychiatry. 1993;150:333–335. doi: 10.1176/ajp.150.2.333. [DOI] [PubMed] [Google Scholar]
- Rycroft N, Hutton SB, Clowry O, Groomsbridge C, Sierakowski A, Rusted JM. Non-cholinergic modulation of antisaccade performance: a modafinil-nicotine comparison. Psychopharmacology. 2007;195:245–253. doi: 10.1007/s00213-007-0885-x. [DOI] [PubMed] [Google Scholar]
- Sailer U, Eggert T, Strassnig M, Riedel M, Straube A. Predictive eye and hand movements are differentially affected by schizophrenia. European Archives of Psychiatry and Clinical Neurosciences. 2007;25:413–422. doi: 10.1007/s00406-007-0749-8. [DOI] [PubMed] [Google Scholar]
- Saslow M. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. Journal of the Optical Society of America. 1967;57:1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Schmid-Burgk W, Becker W, Diekmann V, Jurgens R, Kornhuber HH. Disturbed smooth pursuit and saccadic eye movements in schizophrenia. Archiv fur Psychiatrie und Nervenkrankheiten. 1982;232:381–389. doi: 10.1007/BF00345594. [DOI] [PubMed] [Google Scholar]
- Schulze K, MacCabe JH, Rabe-Hesketh S, Crawford T, Marshall N, Zanelli J, Walshe M, Bramon E, Murray RM, McDonald C. The relationship between eye movement and brain structural abnormalities in patients with schizophrenia and their unaffected relatives. Journal of Psychiatric Research. 2006;40:589–598. doi: 10.1016/j.jpsychires.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Express saccades and smooth pursuit eye movement function in schizophrenic, affective disorder, and normal subjects. Journal of Cognitive Neuroscience. 1993;5:303–316. doi: 10.1162/jocn.1993.5.3.303. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Antisaccades and smooth pursuit eye movements in schizophrenia. Biological Psychiatry. 1995;37:394–401. doi: 10.1016/0006-3223(94)00127-O. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smyrnis N, Malogiannis IA, Evdokimidis I, Stefanis NC, Theleritis C, Vaidakis A, Theodoropoulou S, Stefanis CN. Attentional facilitation of response is impaired for antisaccades but not for saccades in patients with schizophrenia: Implications for cortical dysfunction. Experimental Brain Research. 2004;159:47–54. doi: 10.1007/s00221-004-1931-0. [DOI] [PubMed] [Google Scholar]
- Spengler D, Trillenberg P, Sprenger A, Nagel M, Kordon A, Junghanns K, Heide W, Arolt V, Hohagen F, Lencer R. Evidence from increased anticipation of predictive saccades for a dysfunction of fronto-striatal circuits in obsessive-compulsive disorder. Psychiatry Research. 2006;143:77–88. doi: 10.1016/j.psychres.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Stark L. Abnormal patterns of normal eye movements in schizophrenia. Schizophrenia Bulletin. 1983;9:55–72. doi: 10.1093/schbul/9.1.55. [DOI] [PubMed] [Google Scholar]
- Straube A, Mennicken J, Riedel M, Eggert T, Muller N. Saccades in Gilles de la Tourette’s syndrome. Movement Disorders. 1997;12:536–546. doi: 10.1002/mds.870120410. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Strojwas MH, Mann JJ, Thase ME. Prefrontal and cerebellar abnormalities in major depression: Evidence from oculomotor studies. Biological Psychiatry. 1998;43:584–594. doi: 10.1016/s0006-3223(97)00485-x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Research Reviews. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Ruhrmann S, Brockhaus-Dumke A, Pauli M, Mueller R, Pukrop R, Kosterkotter J. Neural correlates of visuo-spatial attention during an antisaccade task in schizophrenia: An ERP study. International Journal of Neuroscience. 2005;115:681–698. doi: 10.1080/00207450590887475. [DOI] [PubMed] [Google Scholar]
- Thampi A, Campbell C, Clarke M, Barrett S, King DJ. Eye movements and neurocognitive function in treatment resistant schizophrenia: A pilot study. Irish Journal of Psychological Medicine. 2003;20:6–10. doi: 10.1017/S0790966700007448. [DOI] [PubMed] [Google Scholar]
- Tien AY, Pearlson GD, Machlin SR, Bylsma FW, Hoehn-Saric R. Oculomotor performance in obsessive-compulsive disorder. American Journal of Psychiatry. 1992;149:641–646. doi: 10.1176/ajp.149.5.641. [DOI] [PubMed] [Google Scholar]
- Tien AY, Ross DE, Pearlson G, Strauss ME. Eye movements and psychopathology in schizophrenia and bipolar disorder. Journal of Nervous and Mental Disease. 1996;184:331–338. doi: 10.1097/00005053-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Tsunoda M, Kawaski Y, Matsui M, Tonoya Y, Hagino H, Suzuki M, Seto H, Kurachi M. Relationship between exploratory eye movements and brain morphomology in schizophrenia spectrum patients. European Archives of Psychiatry and Clinical Neurosciences. 2005;255:104–110. doi: 10.1007/s00406-004-0540-z. [DOI] [PubMed] [Google Scholar]
- Van der Wee N, Hardeman HH, Ramsey NF, Raemaekers M, van Megen HJ, Denys DA, Westenberg HG, Kahn RS. Saccadic abnormalities in psychotropic-naive obsessive-compulsive disorder without co-morbidity. Psychological Medicine. 2006;36:1321–1326. doi: 10.1017/S0033291706007926. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, VanGisbergen JAM. Skewness of saccadic velocity profiles: A unifying parameter for normal and slow saccades. Vision Research. 1987;27:731–745. doi: 10.1016/0042-6989(87)90071-x. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Rivaud S, Gouider-Khouja N, Pillon B, Bonnet AM, Gaymard B. Eye movements in parkinsonian syndromes. Annals of Neurology. 1994;35:420–426. doi: 10.1002/ana.410350408. [DOI] [PubMed] [Google Scholar]
- Winograd-Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB. Ocular motor differences between melancholic and non-melancholic depression. Journal of Affective Disorders. 2006a;93:193–2003. doi: 10.1016/j.jad.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Winograd-Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB. Self-paced and reprogrammed saccades: Differences between melancholic and non-melancholic depression. Neuroscience Research. 2006b;56:253–260. doi: 10.1016/j.neures.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Winograd-Gurvich C, Fitzgerald PB, Georgiou O, Karistianis N, Millist L, White O. Inhibitory control and spatial working memory: A saccadic eye movement study of negative symptoms in schizophrenia. Psychiatry Research. 2008;157:9–19. doi: 10.1016/j.psychres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Wirtschafter JD, Weingarden AS. Neurophysiology and central pathways in oculomotor control: Physiology and anatomy of saccadic and pursuit eye movements. In: Johnston CW, Pirozzolo FJ, editors. Neuropsychology of Eye Movements. Hillsdale, NJ: Erlbaum; 1988. pp. 5–29. [Google Scholar]
- Wonodi I, Cassady SL, Adami H, Avila M, Thaker GK. Effects of repeated amphetamine administration on antisaccades in schizophrenia spectrum personality. Psychiatry Research. 2006;141:237–245. doi: 10.1016/j.psychres.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye movements and vision. New York: Plenum Press; 1967. [Google Scholar]
- Yee RD, Baloh RW, Marder SR, Levy DL, Sakala SM, Honrubia V. Eye movements in schizophrenia. Investigative Opthalmology and Visual Science. 1987;28:366–374. [PubMed] [Google Scholar]