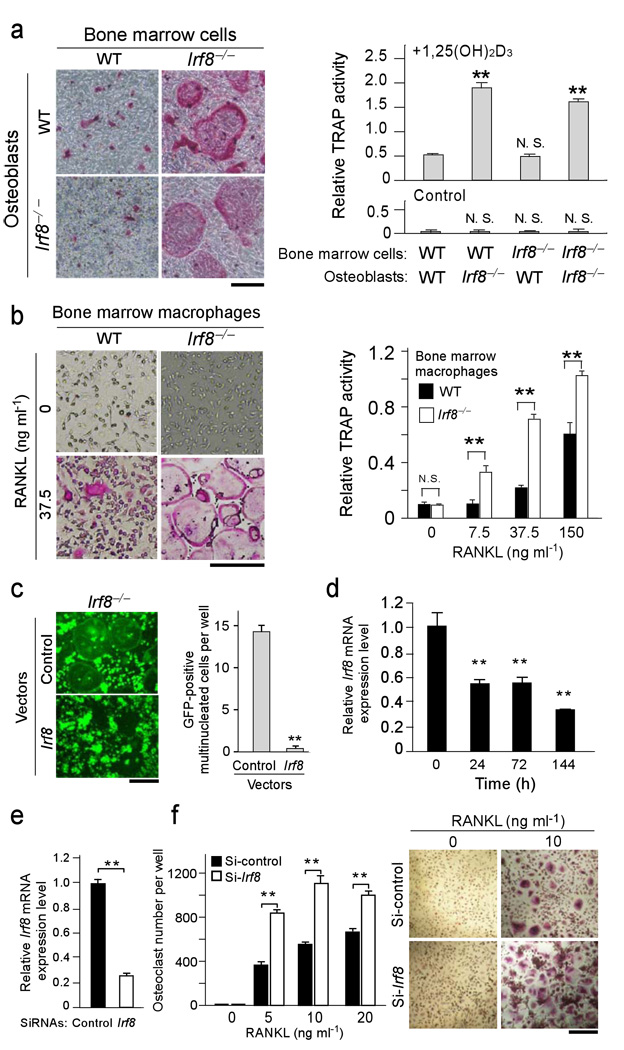
Figure 3.
IRF8 deficiency or RNAi-mediated silencing in osteoclast precursors leads to enhanced osteoclast formation. (a) Primary calvarial osteoblasts and bone marrow cells obtained from wild-type and Irf8−/− mice were cocultured in the presence of 108 M 1,25(OH)2D3 and 10−6 M prostaglandin E2 (inducers of RANKL expression in osteoblasts) for 6 days. TRAP staining (left) and TRAP activity (right) of cultures are shown. Bar, 50 µm. (b) Osteoclast formation induced by 50 ng/ml M-CSF and the indicated doses of RANKL in BMM cultures. TRAP staining (left) and TRAP activity (right) of cultures are shown. Bar, 50 µm. (c) Irf8−/− macrophages were transduced with the vectors such as pMX-Irf8-IRES-EGFP (Irf8) or pMX-IRES-EGFP (Control) and stimulated with 150 ng/ml RANKL for 3 days (left). GFP-positive multinucleated cells were counted as osteoclasts (right). **P<0.01. Bar, 50 µm. (d) Kinetics of Irf8 mRNA expression during human osteoclastogenesis induced by 40 ng/ml RANKL at indicated time points. (e) Human CD14-positive monocytic cells were transfected with human Irf8-specific short interfering RNAs (si-hIrf8) or non-targeting control siRNAs (si-control), cultured for 2 days in the presence of 20 ng/ml M-CSF, and efficiency of silencing of Irf8 mRNA was examined by quantitative real time-PCR. (f) The cells were further stimulated with indicated concentrations of RANKL for 6 days. Number of TRAP-positive multinucleated cells was counted as osteoclasts (left). TRAP-positive cells appear red in the photograph (right). **P<0.01. Representative data from one of three donors is shown; similar results were obtained using a distinct Irf8-specific siRNA in an additional three experiments. Data are expressed as the mean+SD of quad-duplicate cultures. **P<0.01; n.s., no statistical difference. Bar, 50 µm.