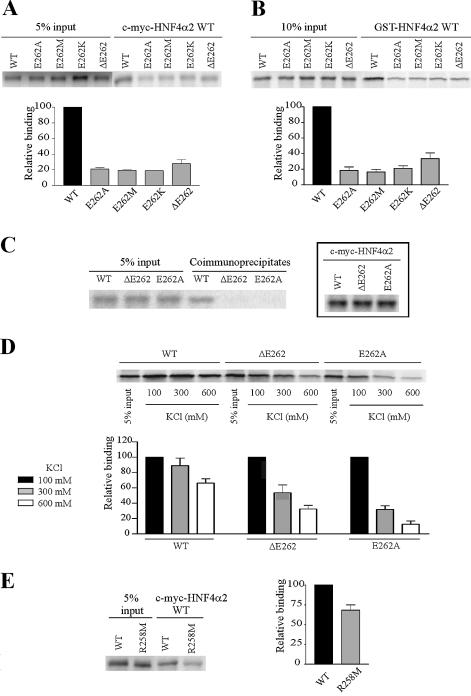
Figure 2.
E262 and R258 are involved in HNF4α2 dimerisation in solution. (A and B) Analyses by co-immunoprecipitation assays and GST pull-down assays, respectively, of dimerisation between immobilised wild-type HNF4α2 fused to c-myc or GST (c-myc-HNF4α2 WT or GST-HNF4α2 WT) and wild-type or mutated [35S]methionine-labelled HNF4α2. Graphs in (A) and (B) indicate means ± SE of HNF4α2 mutant binding relative to that of the wild-type protein from three independent experiments. Inputs were taken into account for binding quantifications. (C) Dimerisation, analysed by co-immunoprecipitation assays of HNF4α2 WT, -ΔE262 or -E262A. For each assay, [35S]methionine-labelled HNF4α2 was incubated with the same protein fused to the c-myc tag. Control of synthesis of c-myc-HNF4α2 WT and mutated proteins is shown in the insert. (D) Dimerisation, analysed by GST pull-down assays of HNF4α2 WT, -ΔE262 and -E262A. For each assay, [35S]methionine-labelled HNF4α2 was incubated with the same protein fused to GST. Pull-down assays were performed in the indicated ionic strength conditions. The graph indicates means ± SE of HNF4α binding at 300 or 600 mM KCl relative to binding at 100 mM KCl (set to 100%) from three independent experiments. (E) Dimerisation, analysed by co-immunoprecipitation assays, between immobilised c-myc-HNF4α2 WT and [35S]methionine-labelled HNF4α2 WT or -R258M. The graph indicates mean ± SE of HNF4α2-R258M binding relative to that of the wild-type protein from four independent experiments. Inputs were taken into account for binding quantifications.