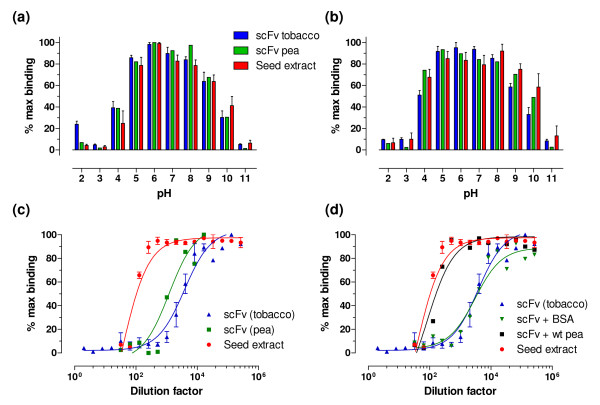
Figure 6.
Analyses of pH and proteolytic stability of scFv AB28 preparations purified either from tobacco leaves or pea seeds and of the AB28-containing pea seed extract. (a, b) pH influence on formation ("binding") and disruption ("dissociation") of the antibody-antigen complexes formed by different AB28-preparations (a and b, respectively). (c, d) Loss of antigen-binding activity of scFv AB28 preparations as a result of degradation by intestinal proteases. (c) Comparison of purified scFv AB28 isolated either from tobacco leaves or from pea seeds as well as of crude protein extract from the scFv-expressing seeds (seed extract). (d) Effect of irrelevant protein (BSA) or wt pea seed extract on proteolytic stability of scFv AB28 (tobacco) in chicken intestinal fluid. For comparison, a degradation curve for AB28-transgenic seed extract is shown. The residual antigen-binding activity was determined by ELISA using the plates coated with the oocyst extract of E. tenella and plotted against the chicken intestinal fluid dilutions.