Abstract
Studies using prostaglandin E receptor (EP) agonists indicate that prostaglandin (PG) E2 can have anabolic effects through both EP4 and EP2 receptors. We previously found that the anabolic response to a selective EP4 receptor agonist (EP4A, Ono Pharmaceutical) was substantially greater than to a selective EP2 receptor agonist (EP2A) in cultured murine calvarial osteoblastic cells. To further define the role of the EP2 receptor in PG-mediated effects on bone cells, we examined the effects of EP2A and PGE2 on both calvarial primary osteoblasts (POB) and marrow stromal cells (MSC) cultured from mice with deletion of one (Het) or both (KO) alleles of the EP2 receptor compared to their wild type (WT) littermates. Deletion of EP2 receptor was confirmed by quantitative real-time PCR, Western Blot and immunohistochemistry. The one month old mice used to provide cells in these studies did not show any significant differences in their femurs by static histomorphometry. EP2A was found to enhance osteoblastic differentiation as measured by alkaline phosphatase mRNA expression and activity as well as osteocalcin mRNA expression and mineralization in the WT cell cultures from both marrow and calvariae. The effects were somewhat diminished in cultures from Het mice and abrogated in cultures from KO mice. PGE2 effects were greater than those of EP2A, particularly in POB cultures and were only moderately diminished in Het and KO cell cultures. We conclude that activation of the EP2 receptor is able to enhance differentiation of osteoblasts, that EP2A is a true selective agonist for this receptor and that PGE2 has an additional anabolic effect likely mediated by the EP4 receptor.
Keywords: Osteoblast, Alkaline Phosphatase, Prostaglandin Receptor, Osteocalcin, Mineralization
INTRODUCTION
Prostaglandins can have both anabolic and catabolic effects on bone [1]. PGE2 is a potent stimulator of osteoblast differentiation. In rat calvarial organ cultures selective agonists for the EP4 and EP2 receptors (EP4A and EP2A) increased bone collagen synthesis, while agonists for EP1 and EP3 receptors as well as other prostanoids were ineffective [2,3]. In osteoblastic cells derived from neonatal mouse calvariae the effects of PGE2 on alkaline phosphatase activity and osteocalcin gene expression could be mimicked by EP4A, while EP2A was less effective [4]. On the other hand EP2A was more effective than EP4A in increasing cAMP production in these cells [5]. The anabolic effects of PGE2 can also be demonstrated in marrow cultures and this was attributed to activation of the EP2 receptor [6]. Endogenous prostaglandins may be important in the differentiation of these cells since deletion of the critical enzyme, inducible cyclooxygenase (COX-2), can result in impairment of differentiation [7, 8]. The present study was undertaken to assess the effects of EP2A and PGE2 on osteoblast differentiation in both marrow stromal cell (MSC) and calvarial primary osteoblast (POB) cultures. The specific role of the EP2 receptor was assessed by comparing cells from wild-type (WT) mice with cells from heterozygotes with deletion of one EP2 receptor allele (Het) and knockouts with deletion of both alleles (KO). Since EP2A can induce COX-2 [5], the studies were carried out in the presence of NS-398, a selective inhibitor of this enzyme, to focus on the direct effects of EP2 receptor activation. EP2A increased alkaline phosphatase mRNA expression and activity as well as OC mRNA expression in both MSC and POB cultures from WT mice by 2 to 5 fold, while the effects were usually reduced in cultures from Het mice and abrogated in cultures from KO mice. EP2A did not enhance mineralization in KO cultures. PGE2 had a greater effect than EP2A, which persisted in cells from Het and KO mice.
MATERIALS AND METHODS
Materials
EP2 receptor agonist (EP2A; AE1-259-01) was from Ono Pharmaceutical (Osaka, Japan). EP2A was tested in CHO cells expressing all EP receptors and showed a binding affinity (Ki) for EP2 receptor of 3.3 nM while for other receptors the Ki was > 6000 nM. The agonist activity (EC50) was 1.8 nM for EP2 receptor and > 10000 for other receptors [9]. EP2 receptor polyclonal antibody, PGE2 and NS-398, a selective inhibitor of COX-2 activity, were purchased from Cayman Chemical Company (Ann Arbor, MI). All other chemicals were from Sigma (St. Louis, MO), unless otherwise noted.
Animals
Mice were in a pure C57BL/6 background. For experiments, males and females with deletion of one EP2 receptor allele (Het) were crossed to obtain KO, Het and WT littermates. EP2 receptor KO mice were initially in C57BL/6 X 129 background {the kind gift of Richard M. Breyer, Vanderbilt University Medical Center, Nashville, 1999, [10]} and were backcrossed more than 9 generations into the C57BL/6 background. Genotyping protocols were followed as described previously [11]. All animal studies were conducted in accordance to the approved protocols by the Animal Care and Use Committee of the University of Connecticut Health Center.
Bone marrow stromal cell (MSC) cultures
Mice were sacrificed at 4–8 wk old. Tibiae and femora were removed aseptically and dissected free of adherent soft tissue. Bone ends were cut off and marrow cavity was flushed with alpha-MEM (Invitrogen, Carlsbad, CA) using a sterile needle. Marrow suspension was dispersed gently by pipetting several times to obtain a single cell suspension. An aliquot of cells, diluted 1:10 in 2% acetic acid, was counted using a haemocytometer. One million cells/well were plated in 6 well dishes in alpha-MEM with 10% heat-inactivated fetal calf serum (HIFCS), penicillin 100 U/ml, streptomycin 50 μg/ml and 50 μg/ml phosphoascorbate (Wako Pure Chemical Industry, Ltd., Osaka, Japan) and cultured in a humidified atmosphere of 5% CO2 at 37° C. Media were changed every 3 d. 10 mM of β-glycerophosphate was added to the culture medium on day 7 for the duration of the experiment. EP2A (1 μM), PGE2 (1 μM), and NS-398 (0.1 μM) were added at the beginning of the cultures. Appropriate vehicle, 0.1% ethanol or 0.1% bovine serum albumin, was added to control cultures.
Primary Calvarial Osteoblast (POB) Cultures
The same mice were used for MSC and POB cultures. Calvariae were dissected from 3–5 mice, washed with PBS and sequentially digested with 0.5 mg/ml of collagenase P (Roche Diagonstics, Indianapolis, IN) in a solution of 1 ml trypsin/EDTA and 4 ml PBS at 37° C with gentle rocking. Five digests were performed, all for 10 min except the last one for 90 min. After each digest, the released cells were collected, the reaction stopped with 10% HIFCS and filtered through a Nitex membrane (Millipore Corp; Bedford, MA) to obtain a single cell suspension. Digests 2–5 were pooled and plated at 5000 cells/cm2 in 6-well dishes in media containing alpha-MEM, 10% HIFCS, penicillin 100 U/ml, streptomycin 50 μg/ml and 50 μg/ml phosphoascorbate. Media were changed every 3 d. 10 mM of β-glycerophosphate was added to the culture medium on day 7 for the duration of the experiment. EP2A (1 μM), PGE2 (1 μM), and NS-398 (0.1 μM) were added at the beginning of the cultures. Appropriate vehicle, 0.1% ethanol or 0.1% bovine serum albumin, was added to control cultures.
Real-time (quantitative) PCR analysis
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions. 3–5 μg of total RNA was DNase treated (Ambion, Inc., Austin, TX) and converted to cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following manufacturer’s instructions. Quantitative PCR for gene expression was performed in 96-well plates using Assays-on-Demand Gene Expression system (Applied Biosystems). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the endogenous control. Each sample was amplified in duplicate. Primers were shown for equal efficiency over a range of target gene concentrations. The PCR reaction mixture (20 μl/well including 2X TaqMan Universal PCR Master Mix, 20X Assays-on-Demand Gene Expression Assay Mix and 50 ng of cDNA) was run in Applied Biosystems ABI Prism 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters. The ΔΔCt method was used for data analysis. A pool of reversed transcribed samples was the calibrator. ΔCt of target = Ct (cycle threshold) of the target in the sample − Ct of GAPDH in the sample, ΔCt of the calibrator = Ct of the target in the calibrator − Ct of GAPDH in the calibrator, and ΔΔCt = ΔCt target − ΔCt calibrator. Relative quantification (RQ, arbitrary units) = 2−ΔΔCt.
Alkaline phosphatase (ALP) activity
Cultures were lysed in 10 mM Tris solution (pH 7.5) supplemented with 0.1% Triton X-100. Supernatants were incubated with an alkaline buffer (pH 10.5) containing 5 mM of p-nitrophenol phosphate as substrate and 2 mM MgCl2. Absorbance was determined at 405 nM and compared with a p-nitrophenol standard titration curve (Sigma). ALP activity was normalized to total protein measured with BCA protein kit (Pierce Chemical Company, Rockford, IL).
Alizarin red staining
To assess mineralization, cells were washed with PBS, fixed in 100% V/V methanol on ice for 30 min and stained with 40 mM alizarin red-S (Sigma) pH 4.2 for 10 min at room temperature. Dishes were washed with water, air dried and scanned for pictures.
Bone histomorphometry
Static histomorphometry was used to evaluate differences among WT, Het and KO mice. Left femurs were dissected free of adherent tissue and fixed in 4% paraformaldehyde in PBS, pH 7.4, at 4°C for 5 days. The femurs were decalcified in EDTA/NH3OH for an additional 5 days, dehydrated in progressive concentrations of ethanol, cleared in xylene, and embedded in paraffin. The embedded femurs were sectioned longitudinally and stained for tartrate-resistant acid phosphatase (TRAP) and counterstained with hematoxylin. Osteoclasts were identified by TRAP staining and by their characteristic multinucleated morphology. Osteoblasts were identified by their characteristic morphology.
Immunohistolabelling
Kidneys were dissected, fixed in paraformaldehyde, and embedded in paraffin. Longitudinal sections were cut and mounted on slides. Slides were cleared in xylene, rehydrated in gradients of ethanol and rinsed with PBS. Slides were incubated with 3% hydrogen peroxide (Sigma) for 30 min, blocked with 5% normal goat serum (NGS) in 1% bovine serum albumin (BSA) in PBS at room temperature for 1 h and then incubated overnight at 4°C with 1:200 of rabbit anti-EP2 antibody (Cayman, Cat No. 101750) in 1% NGS in 1% BSA in PBS. After washing with PBS, slides were incubated with 1:200 goat biotinylated anti-rabbit secondary antibody for 1 hour at room temperature. Slides were developed in vectastain ABC reagent and peroxidase substrate solution (diaminobenzidine and hydrogen peroxide) as per manufacturer’s instruction (Vector Labs, Burlingame, CA), counter stained with hematoxylin, dehydrated and mounted. On all tissues tested, negative controls were performed by omitting the primary antibodies or by applying normal rabbit serum with the same dilution to check for non-specific binding of the secondary antibody.
Western blotting
Proteins were extracted from brain tissue using RIPA buffer following manufacturer’s instructions (Santa Cruz Biotechnology, Santa Cruz, CA). Protein concentrations were determined by BCA protein assay kit (Pierce Chemical Co.). 15 μg of total proteins were separated by 8% SDS-PAGE, and electrotransferred onto nitrocellulose membrane. Membranes were washed with Tris-buffered saline (TBS, pH 7.6), blocked with blocking buffer (1XTBS, 0.1% Tween-20 with 5% w/v non-fat dry milk) and incubated overnight at 4°C with 1:1000 of rabbit anti-EP2 antibody (Cayman, Cat No. 101750) in blocking buffer. After washing with TBS containing 0.1% Tween-20 (TBST), membranes were incubated with HRP-conjugated secondary antibody. After washing again with TBST, the signals were detected by Lumi GLO chemiluminescence reagent (Cell Signaling, Danvers, MA).
Statistics
All values are depicted as the mean ± standard error of the mean (SEM). Statistical analysis was performed using SigmaStat® for Microsoft Windows®, version 2.03 (San Rafael, CA). To compare multiple treatment groups, differences were examined by one-way analysis (ANOVA) followed by the post-hoc Bonferroni test (pairwise multiple comparison).
RESULTS
Receptor Expression and Morphology
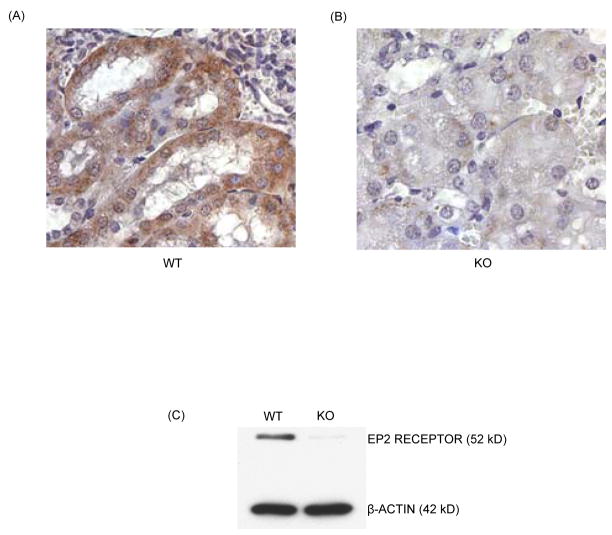
Loss of EP2 receptor expression was confirmed by immunohistochemistry and western blot analysis in two highly expressing tissues, kidney and brain, respectively (Fig. 1). Comparison of the EP2 and EP4 receptor mRNA expression, measured by quantitative real-time PCR showed that EP2 receptor mRNA expression was reduced by 43% (p <0.01) and 25% (p <0.05), respectively in Het MSC after 14 and 21 days of cultures compared to WT cells. EP4 receptor mRNA expression was increased by 50% and 80% (p <0.01), respectively in Het and KO MSC after 21 days of culture (data not shown). Static histomorphometry of 1 month old WT, Het and KO mice showed no significant differences in osteoclast number or osteoblast surface (Table 1). Trabecular bone volume was approximately 60% lower in female than in male mice but not different between genotypes (data not shown).
Figure 1.
EP2 receptor protein expression in kidney and brain tissues of one month old EP2 receptor deletion mice compared to their WT litter mates. Immunohistolabelling in paraffin embedded kidney sections (A) WT and (B) KO in 200 X magnification. C) Western blot analysis in brain tissues was performed as described in materials and methods section.
Effects of EP2A and PGE2 in MSC cultures
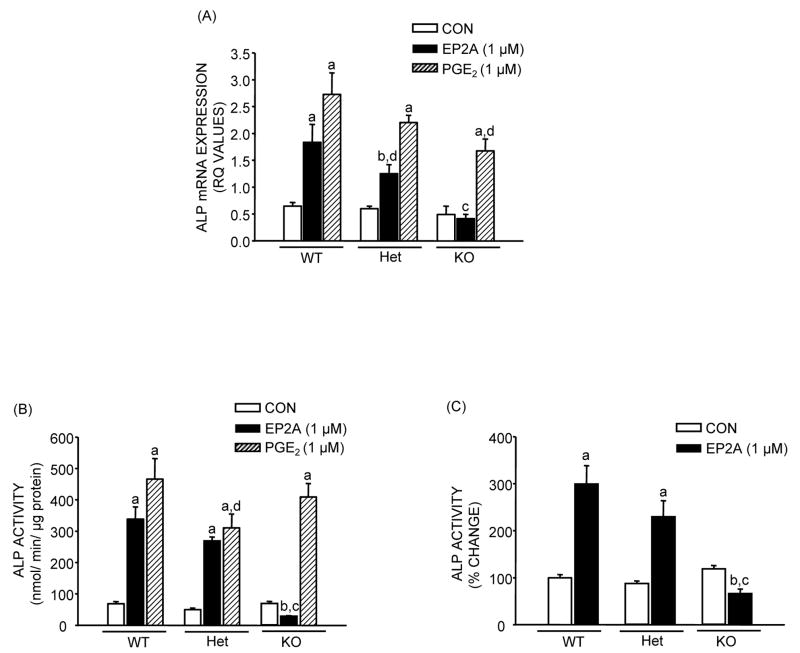
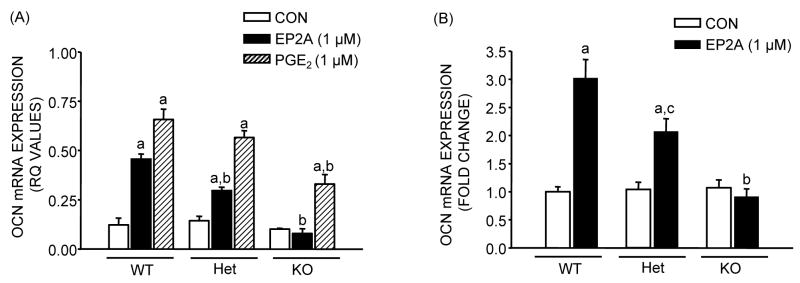
Both EP2A and PGE2 cause significant increases in ALP mRNA expression and activity in MSC cultures after 14 days of culture (Fig. 2). For EP2A the increase in mRNA expression was 2.9 fold (p <0.01) in WT, and 2.1 (p <0.05) fold in Het cultures, but there was no effect in KO cultures. For PGE2 the effects on ALP mRNA were significant for all three genotypes but decreased from 4.3 to 3.7 to 3.4 fold respectively in WT, Het and KO cultures (Fig. 2A). The effect of EP2A on ALP activity was not significantly decreased in Het cultures compared to WT but was actually inhibitory in KO cultures (Fig. 2B and C). The effects of PGE2 on ALP activity were not diminished in Het and KO cultures (Fig. 2B). The effects of EP2A and PGE2 on OCN mRNA expression were similar to the effects on ALP mRNA expression (Fig 3). EP2A increased mRNA levels 3.8 fold (p <0.01) in WT, 2.2 fold (p <0.01) in Het and had no effect in KO cell cultures. The effects of PGE2 were larger and significant in all three genotypes but showed some decrease from 5.5 fold in WT to 4.0 fold in Het and 3.3 fold in KO cultures (Fig. 3A). The results were similar when 3 separate experiments were pooled (Fig. 3B). Deletion of EP2 receptors decreased mineralization (Fig. 4) both in control and EP2A treated cultures but had little effect in PGE2 treated cultures.
Figure 2.
Effects of EP2A (1 μM) and PGE2 (1 μM) on alkaline phosphatase (ALP) mRNA expression and activity in MSC after 14 days of culture from EP2 receptor WT, Het and KO mice in the presence of NS-398 (0.1 μM). (A) ALP mRNA expression. Analysis for mRNA expression was done by quantitative real-time PCR. Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) served as the internal control. Relative quantification (RQ) of target gene expression in a sample to a control calibrator sample (ΔΔCt method) was used for data analysis. (B) ALP activity normalized to total protein. (C) Percentage change in ALP activity relative to WT control after pool of 3 independent experiments. Each bar is the mean ± SEM of n = 3–18 samples. aSignificant effect of treatment, p <0.01; bp <0.05. cSignificant effect of genotype, p <0.01; dp <0.05.
Figure 3.
Effects of EP2A (1 μM) and PGE2 (1 μM) on osteocalcin (OCN) mRNA expression in MSC after 21 days of culture from EP2 receptor WT, Het and KO mice in the presence of NS-398 (0.1 μM). Analysis for mRNA expression was done by real-time PCR using relative quantification (ΔΔCt method). (A) OCN mRNA expression (relative quantification values). (B) Fold change in OCN mRNA expression relative to WT control after pool of 2 independent experiments. Each bar is the mean ± SEM of n = 3–6 samples. aSignificant effect of treatment, p <0.01. bSignificant effect of genotype, p <0.01; cp <0.05.
Figure 4.

Alizarin red staining for osteoblast mineralization in MSC after treatment with EP2A and PGE2 for 21 days from EP2 receptor WT, Het and KO mice in the presence of NS-398 (0.1 μM).
Effect of EP2A and PGE2 in POB cultures
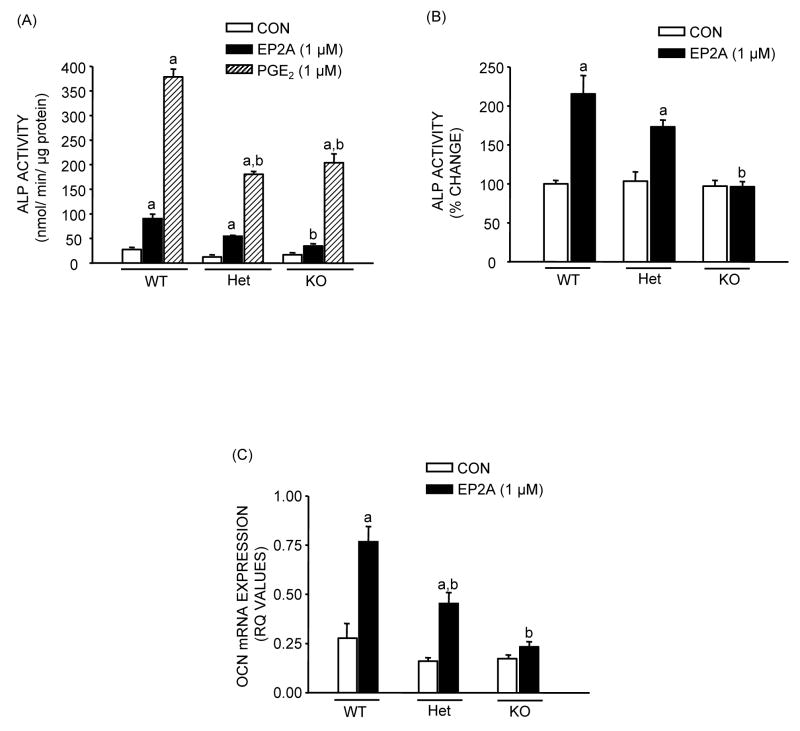
The effects of deletion of the EP2 receptor on EP2A responses were similar in POB cultures to those seen with MSC (Fig. 5). However there appeared to be a greater relative effect of PGE2, which increased ALP activity by 10–15 fold (Fig. 5A). Pooled data showed a pattern of response to EP2A that was somewhat smaller than for MSC cultures with ALP activity increasing 2 fold (p <0.01) in WT, 1.7 fold (p <0.01) in Het, with no effect in KO cultures (Fig. 5B). A decrease in protein content of 39–45% was seen with EP2A in WT and Het cultures with no effect in KO cultures (data not shown). After 21 days in culture, osteocalcin mRNA (Fig. 5C) showed a significant 2.8 fold (p <0.01) increase with EP2A in WT and a similar relative increase in Het POB cultures with no significant effect in KO cell cultures.
Figure 5.
Effects of EP2A (1 μM) and PGE2 (1 μM) treatment on markers of osteoblastic differentiation in POB cultures from EP2 receptor WT, Het and KO mice in the presence of NS-398 (0.1 μM). (A) ALP activity, normalized to total protein at 14 day. (B) Percentage change in ALP activity relative to WT control after pool of 3 independent experiments. (C) OCN mRNA expression at the end of 21 days of culture. Each bar is the mean ± SEM of n = 3–18 samples. aSignificant effect of treatment, p <0.01. bSignificant effect of genotype, p <0.01.
DISCUSSION
Our results clearly demonstrate that selective activation of the EP2 receptor can enhance differentiation of cells of the osteoblastic lineage. We found that the effects of the selective agonist EP2A were variably diminished in cells from Het mice, and abrogated in cells from KO mice, while the effects of PGE2 were not significantly decreased. Loss of the EP2 receptor was confirmed by real-time PCR, Western Blot and immunocytochemistry in the KO animals. However in vivo osteoblast surface and osteoclast numbers were not affected by EP2 receptor deletion. The effects of EP2A relative to that of PGE2 appear to be larger in MSC than in POB. This may be because MSC contains less differentiated precursors which are more responsive to EP2A. The greater relative effect of PGE2, compared to EP2A in POB cultures confirms our previous findings. The persistent effects of PGE2 in EP2 receptor KO cells presumably reflect an effect mediated by activation of the EP4 receptor which we have shown to stimulate osteoblast differentiation and function [3,4]. A small increase in EP4 receptor mRNA expression in the EP2 KO cells suggests that this may be a compensatory mechanism that could operate in vivo and explain the absence of histologic changes in the bones of EP2 receptor KO animals.
The present studies can provide a model for mechanistic studies of the pathways by which EP2 and EP4 receptor activation induce osteoblast differentiation. Based on our previous studies showing that EP2A is more effective than EP4A in increasing cAMP production [5] it seems likely that some of the effects on differentiation could be mediated by another pathway. For example it has been recently suggested that EP4 receptor activation could act through the PI3 kinase pathway, which is known to be important in the response to growth factors [12].
While EP4 activation may be more effective at increasing osteoblast differentiation, activators of the EP2 receptor may still prove useful clinically. A different selective EP2 receptor agonist has recently been shown to enhance healing of bone defects and increase bone formation when injected locally [13]. Moreover EP2 receptor activation may have the advantage, based on our previous studies in rat organ cultures [3], that it has relatively less stimulatory effect on bone resorption than EP4 activation.
Acknowledgments
This work was funded by NIH Grants AR18063 (Raisz) and DK48361 (Pilbeam)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pilbeam CC, Harrison J, Raisz LG. Prostaglandins and bone metabolism. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2. San Diego, CA, USA: Academic Press; 2002. pp. 979–94. [Google Scholar]
- 2.Woodiel FN, Fall PM, Raisz LG. Anabolic effects of prostaglandins in cultured fetal rat calvariae: structure-activity relations and signal transduction pathway. J Bone Miner Res. 1996;9:1249–55. doi: 10.1002/jbmr.5650110909. [DOI] [PubMed] [Google Scholar]
- 3.Raisz LG, Woodiel FN. Effects of selective prostaglandin EP2 and EP4 receptor agonists on bone resorption and formation in fetal rat organ cultures. Prostaglandins Other Lipid Mediat. 2003;71:287–92. doi: 10.1016/s1098-8823(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 4.Alander CB, Raisz LG. Effects of selective prostaglandins E2 receptor agonists on cultured calvarial murine osteoblastic cells. Prostaglandins Other Lipid Mediat. 2006;81:178–83. doi: 10.1016/j.prostaglandins.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuma Y, Li Z, Pilbeam CC, Alander CB, Chikazu D, Kawaguchi H, Raisz LG. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E(2) and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone. 2004;34:827–34. doi: 10.1016/j.bone.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Scutt A, Zeschnigk M, Bertram P. PGE2 induces the transition from non-adherent to adherent bone marrow mesenchymal precursor cells via a cAMP/EP2-mediated mechanism. Prostaglandins. 1995;49:383–95. doi: 10.1016/0090-6980(95)00070-q. [DOI] [PubMed] [Google Scholar]
- 7.Chikazu D, Li X, Kawaguchi H, Sakuma Y, Voznesensky OS, Adams DJ, Xu M, Hoshi K, Katavic V, Herschman HR, Raisz LG, Pilbeam CC. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfa1 binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J Bone Miner Res. 2005;20:1888–98. doi: 10.1359/jbmr.2005.20.10.1887. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Choudhary S, Okada Y, Voznesensky O, Alander C, Raisz L, Pilbeam C. Cyclooxygenase-2 gene disruption promotes proliferation of murine calvarial osteoblasts in vitro. Bone. 2007;41:68–76. doi: 10.1016/j.bone.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto H, Maruyama T, Sakata K, Koketsu M, Kobayashi M, Yoshida H, Seki A, Tani K, Maruyama T, Kondo K, Ohuchida S. Novel four selective agonists for prostaglandin E receptor subtypes. Prostaglandins and other lipid mediators. 1999;59:1–235. (Abstract) [Google Scholar]
- 10.Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CR, Breyer RM, Raisz LG. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology. 2000;141:2054–61. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- 12.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–6. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 13.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, Owen TA, Li M, DaSilva-Jardine P, Zhou M, Dunn RL, Dumont F, Korsmeyer R, Krasney P, Brown TA, Plowchalk D, Vukicevic S, Thompson DD. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100:6736–40. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]