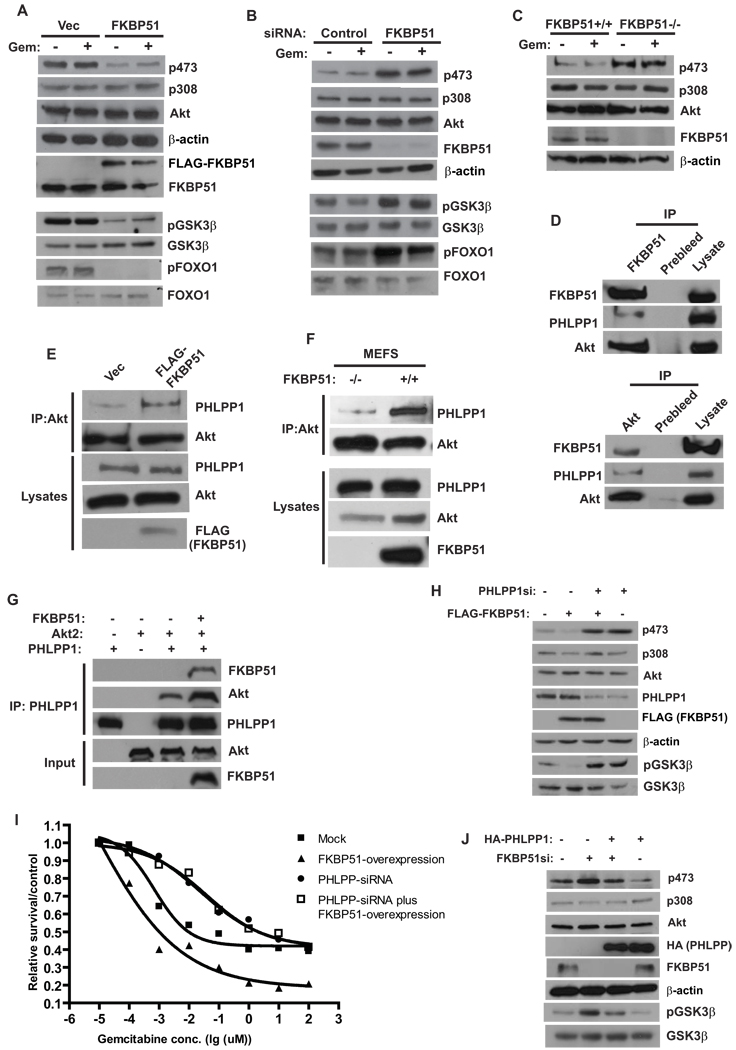
Figure 2. FKBP51 regulates Akt phosphorylation at Ser473 by promoting Akt-PHLPP interaction.
(A). SU86 cells were transfected for 48 hr with indicated constructs. Cells were treated with DMSO or gemcitabine (Gem, 20 nM, 12 hrs), and the phosphorylation of Akt, FOXO and GSK-3β in cell lysates was detected by Western Blot. (B). SU86 cells were transfected with indicated siRNA. Cells were then treated and harvested as in (A). (C). The phosphorylation of Akt in cell lysates from FKBP51+/+ or FKBP51−/− cells was examined. (D). The coimmunoprecipitation of Akt, PHLPP and FKBP51 was examined. (E). 293T cells were transfected with indicated constructs, and the interaction between Akt and PHLPP was examined. (F). The PHLPP-Akt interaction was examined in FKBP51+/+ and FKBP51−/− cells. (G). Purified recombinant Akt, PHLPP1 and FKBP51 were incubated in vitro as indicated. The Akt-PHLPP interaction was then examined by coimmunoprecipitation. (H–I). SU86 cells were transfected with FLAG-FKBP51 and/or PHLPP siRNA as indicated. The phosphorylation of Akt, GSK-3β, as well as sensitivity to gemcitabine were then examined. Points, mean values for three independent experiments; Error bars, +/− SEM. (J). SU86 cells were transfected with HA-PHLPP and/or FKBP51 siRNA as indicated. The phosphorylation of Akt and GSK-3β was then examined.