Figure 2. Structures of native and FimH-bound mouse uroplakin particles at 8 Å resolution.
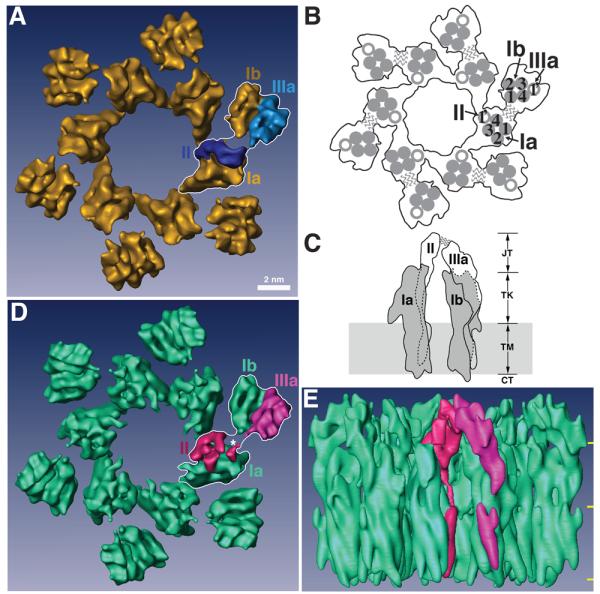
(A) Top view of the native 16-nm particle, presented as the reference structure. One of the 6 subunits is outlined by a white line stroke with its individual uroplakins labeled. Each subunit consists of an inner and an outer subdomain that contains the uroplakins pairs Ia/II and Ib/IIIa, respectively. Bar scale: 2 nm. This scale also applies to panels (D) and (E). (B) Schematic top view of the 16-nm particle showing the relative positions of the UP transmembrane helices. In one subunit, the transmembrane helices of the tetraspanin UPs Ia and Ib are numbered. The wavy lines indicate the weak connection between the joint regions of UPs II and IIIa that link the inner and outer subdomains within each subunit. (C) Schematic side view of a subunit of the 16-nm particle. The tetraspanin UPs Ia and Ib are shown in grey, while the single-pass UPs II and IIIa are shown in white. Dashed lines indicate the portions of the structures obscured by the molecules in front. The wavy lines again indicate the weak joint connection between the two subdomains. In the vertical dimension the particle can be divided into four regions, from top to bottom: the joint (JT), the trunk (TK), the transmembrane domain (TM), and the cytoplasmic tail (CT). Previous modeling depicted the UPs Ia and Ib as cylindrically shaped structures with a total height of ∼9 nm (CT+TM+TK).14,62 (D) Top view of the FimH-bound 16-nm particle. As in panel (A), one subunit is outlined by a white line stroke and its UPs II and IIIa are rendered in red and magenta, respectively. The asterisk points to the FimH binding site (see text). Note the considerable conformational differences in UPs II and IIIa when compared with the native structure in (A). (E) Side view of the FimH-bound 16-nm particle colored as in panel (D). The three horizontal yellow marks on the right demarcate the 4 regions explained in panel (C).
