Figure 3. Comparisons of the subdomain structures of native and FimH-bound uroplakin receptor complexes.
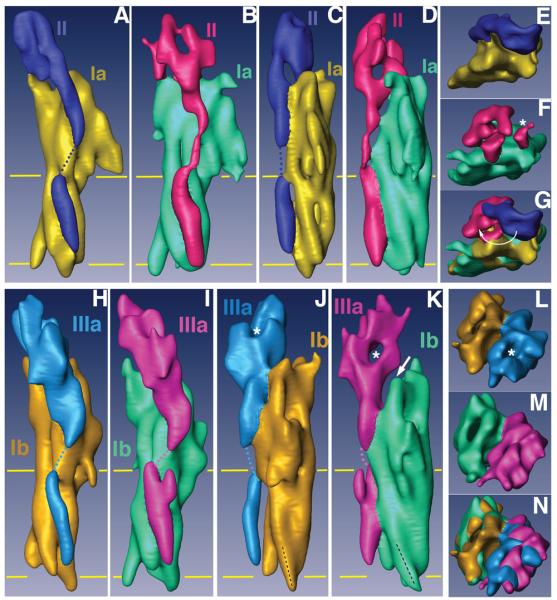
(A)-(G) represent different views of the UP Ia/II pair of the native and FimH-bound particles, and (H)-(N) the comparable views for UP Ib/IIIa pair. The individual uroplakins are segmented and labeled. The horizontal yellow lines mark the TM region. (A) and (B) Side views of the UP Ia/II pair in the inner subdomains of native (A) and FimH-bound (B) particles. (C) and (D) Comparison of the same UP Ia/II pair as in (A) and (B) but rotated ∼90°. Note the drastic FimH binding-induced shape change in the joint domain of UP II. Also note the connecting density in the TK of UP II (B and D) as a result of FimH-binding.14 (E) and (F) Top views of the UP Ia/II pair in the inner subdomains of native (E) and FimH-bound (F) particles. The asterisk in (F) indicates the FimH binding site of UP Ia. (G) Superimposed top views of the UP Ia/II structures shown in (E) and (F). Note the large swing of the joint domain of UP II indicated by a curved arrow. (H) and (I) Side views of the outer subdomain UP Ib/IIIa pair of the native (H) and FimH-bound (I) particles. (J) and (K) Comparison of the same UP Ib/IIIa pair as in (H) and (I) but rotated ∼90°. Note in (J) and (K) the orientation (black dashed line) of the cytoplasmic end of UP Ib helix 3 changed after FimH binding. Also note the separation (arrow in panel K) between UPs Ib and IIIa resulting from the FimH-induced conformational changes. (L) and (M) Top views of the outer subdomains of the native (L) and FimH-bound (M) particles. (N) Top view of the outer subdomains of the two structures superimposed.
