Figure 2. Cathepsin L and prohormone convertases: predicted proteolytic pathways for CCK and neuropeptide production.
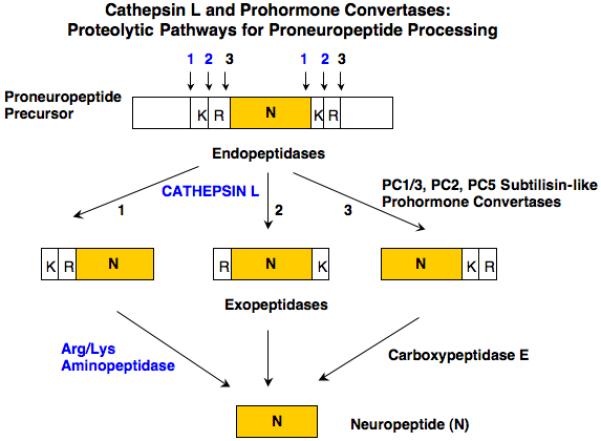
Dual protease pathways are proposed for proneuropeptide processing consisting of the cysteine protease cathepsin L with aminopeptidase B (AP-B), and the subtilisin-like prohormone convertases (PC1/3, PC2, and related PCs) combined with carboxypeptidase E (CPE). Studies have shown differences in cleavage specificities among these proteases. Cathepsin L prefers to cleave at the NH2-terminal side, and between, dibasic residue processing sites. The PC enzymes prefer to cleave at the COOH-terminal side of dibasic residues. These cleavage specificities for the cathepsin L and PC pathways are predicted to each yield mature neuropeptides such as CCK8.
