Abstract
The objective of this study was: (1) to characterize the P-gp inhibitory effect of different concentrations of Pluronic P85 on anti-HIV-1drug cellular accumulation, and (2) to investigate the relationship between cellular accumulation and free fraction of drug. Cellular accumulation studies in MDCKII-WT and MDCKII-MDR1 cell monolayers showed a biphasic dose response characterized by decline in accumulation at Pluronic concentrations greater than the CMC. This phenomenon was independent of the inhibition of P-gp efflux by Pluronic. Cell-free equilibrium dialysis was used to determine the effect of Pluronic P85 on drug free fraction and the affinity of Pluronic micelles for drug was modeled. Nelfinavir and saquinavir associated extensively with micelles and equilibrium free fractions were low at P85 concentrations above the CMC, with association constants being in the order nelfinavir > saquinavir >>> abacavir. Abacavir, a P-gp substrate, showed no association with micelles yet showed a biphasic response in cellular accumulation. These data suggest that, above the CMC, inhibition of P-gp is not affected but rather factors such as micellar trapping could contribute to decreased accumulation. Therefore, the in vitro evaluation of the effect of Pluronic formulations on active transport should take into account both the physicochemical properties of drug and the composition of Pluronic.
Keywords: Efflux pumps, HIV-1 protease inhibitors, Pluronics, polymers, critical micellar concentration, P-glycoprotein, equilibrium dialysis, micelle
Introduction
Surfactants have conventionally been used as stabilizing excipients because of their ability to lower interfacial energy and thereby stabilize formulations1. Surfactants can enhance the solubility of lipophilic drugs, thereby increasing absorption in the gut, an example being the solubilization of the anti-cancer agent paclitaxel using cremophor EL 2. Typically, excipients used in pharmaceutical preparations have been considered to be inert and nonreactive but contrary to this, nonionic surfactants such as cremophor EL, Solutol, Tweens, Spans and vitamin E TPGS have been reported to have biological activity against P-gp-mediated efflux of drugs 3–5 and recently many of the same excipients have been shown to inhibit the efflux transporter BCRP 6.
Drug efflux transporters are transmembrane proteins that are instrumental in the efflux of a variety of structurally diverse drugs out of cells using the energy generated from ATP hydrolysis 7. The efflux activity of P-gp, in particular, has been implicated in reducing the exposure of a number of drugs such as anti-cancer agents and anti-HIV1 drugs, to the systemic circulation as well as to important target tissues such as the brain 8,9 and inhibition of P-gp activity has been shown to improve the exposure of drugs systemically and in target tissues 8,10.
Amphiphilic block copolymers are another group of compounds typically used as excipients for solubilization and delivery of drugs 11–13. These nonionic block copolymers have been considered suitable for use as drug delivery vehicles due to their ability to form micelles in the nanometer range, with the advantage of greater thermodynamic and kinetic stability over conventional surfactants 14.
Some amphiphilic block copolymers have been reported to have the ability to sensitize active drug efflux transporters. Methoxypolyethylene-block-polycaprolactone (MePEG-b-PCL) diblock copolymers have been reported to enhance the cellular accumulation of rhodamine 123 in caco-2 cells by inhibiting P-gp 15 and polyethylene oxide (PEO)-polypropylene oxide (PPO) block copolymers (Pluronics) have inhibitory activity against multiple drug efflux transporters 16,17.
Pluronics are amphiphilic in nature and possess an A-B-A configuration with the hydrophobic polypropylene oxide (B) block flanked by the hydrophilic polyethylene oxide (A) blocks 18. The relative length of the hydrophilic and hydrophobic blocks determines properties such as the critical micellar concentration (CMC), aggregation number and shape of the micelle. Pluronic micelles have a hydrodynamic diameter ranging from 20 – 80 nm allowing for prolonged circulation time inside the body 18,19. Pluronic P85 (PEO39-PPO52-PEO39), an amphiphilic block copolymer, has been reported to possess inhibitory activity against multiple drug efflux transporters. In vitro Pluronic P85 increased membrane and transcellular transport of many drugs in cultured caco-2 monolayers and primary cultures of bovine brain microvessel endothelial cells (BBMEC) 20,21 as well as in cells genetically modified to over-express drug efflux transporters like P-gp and MRPs 16,17. In vivo, Pluronic P85 has been shown to significantly enhance the brain penetration of the P-gp substrate digoxin by inhibiting P-gp at the blood-brain barrier 16.
The inhibition of P-gp by amphiphilic block copolymers has been reported to be a function of the concentration of the block copolymer used. MePEG-b-PCL diblock copolymers increased rhodamine 123 accumulation in caco-2 cells at concentrations 10-fold higher than at their CMC 22 while Pluronic block copolymers, on the other hand, enhanced rhodamine 123 accumulation in caco-2 and BBMEC monolayers up to the CMC followed by a decline in cellular accumulation 23,24. In our laboratory, we have previously investigated the ability of Pluronics to modulate the P-gp mediated active efflux of HIV-1 protease inhibitors using the human MDR1-transfected MDCKII cell model that over-expresses human P-gp25. HIV-1 protease inhibitors are hydrophobic drugs that may associate with the hydrophobic core of Pluronic micelles at concentrations above the CMC. It has been reported that Pluronic P85 has a prolonged circulation time in the blood when injected at micellar concentrations in mice, suggesting that Pluronic micelles might exist for a long time in the body 19. This could have implications for cellular delivery of hydrophobic P-gp substrates that are liable to associate with the hydrophobic core of the micelles.
Therefore, the objective of this study was to investigate the effect of the block copolymers Pluronic P85, F127 and F88 on P-glycoprotein inhibition and subsequent cellular accumulation of HIV1 agents as a function of Pluronic concentration, using in vitro cell accumulation studies. Subsequently, the study aimed to examine the possible correlation between the effects observed in the biological cell-based system with the effect of micellar concentrations of Pluronic on drug free fraction using an in vitro cell-free equilibrium dialysis system.
MATERIALS AND METHODS
Cell lines
Wild-type (WT) and MDR1-transfected epithelial Madin-Darby canine kidney (MDCKII) cells were obtained from Dr. Piet Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands). The MDCKII cells were maintained in Dulbecco's modified eagle medium (Mediatech, Inc., Herndon, VA) fortified with 10% heat deactivated fetal bovine serum (SeraCare Life Sciences, Inc., Oceanside, CA). The media was fortified with 100 U/mL of penicillin and 100 μg/mL of streptomycin (Sigma Aldrich, St.Louis, MO) and the cells were incubated at 37°C under humidity and 5% CO2 tension. The MDCKII-MDR1 cell growth media additionally contained 80 ng/mL of colchicine to maintain positive selection pressure for P-gp expression. Cells between passages 5 and 15 were used in all experiments.
Chemicals
[3H]-vinblastine (specific activity: 6 Ci/mmoL), [14C]-nelfinavir (specific activity: 60 mCi/mmoL), [3H]-saquinavir (specific activity: 1.9 Ci/mmoL), [14C]-mannitol (specific activity: 53 mCi/mmoL) and [3H]-abacavir (specific activity: 0.7 Ci/mmoL) were obtained from Moravek Radiochemicals (Brea, CA) and [14C]-nelfinavir (specific activity: 0.7 Ci/mmoL) was a gift from Pfizer (New York, NY). Pluronics P85, F127 and F88 were a gift from the BASF Corporation (Florham Park, N.J.).
Cellular accumulation studies in MDCKII cells
For the accumulation experiments, cells were seeded in clear polyester 12-well plates (TPP© cell culture plate) at a seeding density of 2×105 cells/well. The media was changed every other day and the cells formed confluent monolayers in 4 days. On the day of the experiment, the media was aspirated and the confluent monolayer was washed twice with prewarmed (37°C) assay buffer (NaCl, 122 mM; NaHCO3, 25 mM; Glucose, 10 mM; HEPES, 10 mM; KCl, 3 mM; MgSO4·7H20, 1.2 mM; CaCl2·H20, 1.4 mM; K2HPO4, 0.4 mM; pH of 7.4). The cells were preincubated for 30 min with 1 mL of assay buffer, after which the buffer was aspirated and the cells were exposed to a tracer solution of radiolabeled drug in 1 mL assay buffer per well. The plates were continuously agitated at 60 rpm in an orbital shaker and maintained at 37°C for the duration of the experiment. Following a 3-hour accumulation period the supernatant was aspirated and the cells were first washed three times with 2 mL of ice cold phosphate buffered saline (PBS) and then solubilized using 1mL of mammalian protein extraction reagent (MPER; Pierce Biotechnology, Inc., Rockford, IL). A 200μL sample was drawn from each well in triplicate and 4 mL of scintillation fluid (ScintiSafe Econo1 cocktail; Fisher Scientific Co.) was added to each sample and counted using liquid scintillation counting (LS-6500, Beckman Coulter, Inc., Fullerton, CA) to determine the radioactivity associated with the cells. Accumulated drug was normalized to cellular protein using the BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL).
For polymer dose response studies, the cells were treated with 0, 0.001, 0.005, 0.01, 0.03, 0.05, 0.1 and 0.5 % w/w of Pluronics P85, F127 or F88. Dose response studies with a small molecule P-gp inhibitor were conducted using 0, 0.07, 0.15, 0.31, 0.62, 1.25, 2.5 and 5μM GF120918, both during the preincubation and the accumulation periods. Drug accumulation in WT and MDR1 cells is expressed as the amount of radioactivity accumulated per milligram of protein. The 1% w/w stock solutions for all the Pluronics were prepared in distilled water while the stock solution for GF120918 was prepared in DMSO. All the stocks were diluted to desired concentrations using assay buffer. The cells were exposed to less than 0.1% DMSO in the assay buffer.
Modeling the effect of GF120918 on nelfinavir accumulation
The sigmoid Emax model with baseline was fit to the data for the effect of GF120918 on cellular accumulation in both WT and MDR1 cells,
| (Equation 1) |
where E0 is the cellular accumulation in the absence of GF120918, Emax is the maximal effect of GF120198 on nelfinavir accumulation, EC50 is the concentration of GF120918 that increases cellular accumulation of nelfinavir to 50% of its maximum, C is the concentration of GF120918 in μM and γ is the shape factor. The pharmacodynamic module in WinNonlin (version 5.2, Pharsight Corp. Mountain View, CA) was used to fit the effect model to the data with equal weighting, and the estimates of Emax, EC50, E0 and γ were obtained.
Determination of the critical micellar concentration for Pluronic P85
The CMC of Pluronic P85 was measured using the fluorescent probe 1, 6- diphenyl-1, 3, 5-hexatriene (DPH). DPH has been used previously for the determination of CMC26. DPH is a fluorescent molecule that due to its hydrophobic nature will incorporate readily into the hydrophobic core of Pluronic P85 micelles. The change in the environment of the probe from aqueous solution to the hydrophobic core of the micelle results in a significant increase in fluorescence emission intensity. Thus, formation of micelles at the CMC will result in an increase in fluorescence that can be measured as an abrupt change in the slope of the fluorescence intensity versus P85 concentration curve.
Different concentrations of Pluronic P85 in assay buffer were incubated with 10 μM DPH, in triplicate, for 24 hrs at 37°C. The samples were then analyzed for fluorescence intensity using a FL600 fluorescence micro-plate reader (Biotek Instruments, Winooski, VT) at an excitation wavelength of 360 nm and emission wavelength of 460 nm. Based on the change in fluorescence intensity observed, the concentrations of P85 were separated into two groups and the data in each group were fit using linear regression. The concentration of Pluronic P85 at which the two regression lines intersected was determined to be the CMC.
Equilibrium dialysis studies
Equilibrium dialysis was conducted using four separate dialysis units per experiment. Each dialysis unit consisted of a donor (2mL) and receiver (2mL) compartment separated by a semi-permeable Spectra/Por regenerated cellulose membrane (Spectrum Laboratories, Rancho Dominguez, CA) with a MWCO of 50,000 Daltons. The entire system was maintained at 37°C by a re-circulating water bath and stirred continuously with magnetic stir-bars to maintain homogeneity. A known amount of radiolabeled drug in 2 mL assay buffer was placed in the donor compartment with an equal volume of fresh assay buffer placed in the receiver compartment. A 10μl sample was drawn from both the donor and receiver chambers at predetermined time points and immediately replaced with fresh assay buffer. The samples were analyzed using liquid scintillation counting (LS 6500, Beckman Coulter, Inc., Fullerton, CA). The dialysis system was considered to have achieved equilibrium if the sampled counts in both the donor and receiver did not change over three consecutive measurements, each spaced hours apart and mass balance was achieved.
Nelfinavir, saquinavir and abacavir equilibrium free fractions were determined as a function of Pluronic P85 concentration. Mannitol equilibrium dialysis was conducted in the absence of Pluronic P85 and at the micellar concentration of 0.1% w/w P85. For concentrations of Pluronic P85 lower than the CMC (0, 0.001, 0.005, 0.01, 0.03 % w/w), Pluronic P85 solution in assay buffer was added to both the donor and receiver compartments since the MWCO of the membrane used (50,000 daltons) was 10-fold greater than the molecular weight of Pluronic P85 unimers (4600 daltons). In case of micellar concentrations of P85 (0.05, 0.1, 0.25, 0.5 % w/w), a 0.025% w/w solution of Pluronic P85 was added to the receiver and the donor received the desired micellar concentration plus 0.005% w/w Pluronic P85. For example, for a desired concentration of 0.05% w/w P85 in donor, a 0.025% w/w Pluronic solution was added to the receiver while the donor received 0.055% w/w P85 such that over time the unimer concentration in the receiver would reach 0.03%w/w (CMC). This may be explained by the ability of unimers to cross the membrane thereby preventing the possibility of micelle formation in the receiver compartment. The free fraction of drug at a given concentration of Pluronic P85 was calculated using the following equation,
| (Equation 2) |
Equilibrium dialysis modeling
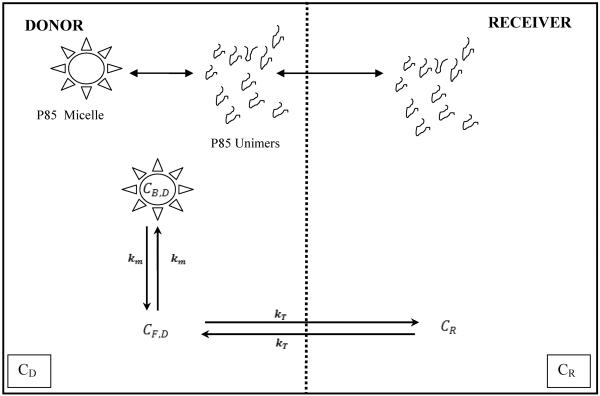
A two-compartment closed system was used to model the equilibrium dialysis of drugs with Pluronic P85. The model consisted of a donor and a receiver compartment with unbound drug being able to cross the membrane. The transfer rate constant from the donor to the receiver and vice versa was modeled to be the same and was estimated from the fit of the model to the data. Pluronic P85 unimers were modeled to be able to cross the membrane (MWCO of membrane was 10 times larger than the unimer molecular weight) while the micelles could not cross from the donor to the receiver compartment due to their large size. In the donor compartment, drug could exist either in a free state or associated with micelles. It was assumed that the drug was not associated with P85 unimers. Figure 1 provides a schematic of the model used and the total concentrations in the donor and receiver are given by the following equations;
| Equation 3 |
| Equation 4 |
where Cd and Cr are the donor and receiver concentrations at any time t, Cd0 is the donor concentration at time t = 0, kt is the transfer rate constant of drug across the semi-permeable membrane and is considered to be the same in both directions, and K is defined as the ratio of free drug to free plus bound drug in the donor compartment and is bound by the limits of 0 and 1. The model was fitted simultaneously to both the donor and receiver concentrations to obtain the transfer rate constant, kt. The effective permeability of drug across the semi-permeable membrane was calculated as,
| Equation 5 |
where V is the volume of the donor or receiver (2 cm3) and A is the area of the membrane (0.6356 cm2) and kt is the transfer rate constant (1/sec). The Peff was expressed in cm/sec. The affinity of drug for Pluronic P85 micelles was calculated using the following equation,
| Equation 6 |
Where fu is the free fraction of the drug, (X-CMC) is the concentration of Pluronic P85 micelles and is obtained by subtracting the CMC of Pluronic P85 (0.03% w/w) from concentration of P85 used. Finally, ka is the affinity rate constant for the affinity of drug for the hydrophobic micellar core.
Figure 1.
Figure 1 presents a schematic of the equilibrium dialysis model describing drug transport across a semi-permeable membrane and the influence of different concentrations of Pluronic P85 on distribution of drug between the donor and receiver compartments. CD, denotes the total concentration of drug in the donor compartment and is formed of the free and bound drug. The free drug concentration in the donor compartment is denoted by CF,D and the bound concentration is denoted as CB,D. The concentration of drug in the receiver compartment is denoted as CR. Bound drug is associated with Pluronic micelles that cannot cross the membrane into receiver. The Pluronic unimers and unbound drug can freely cross the membrane. The transfer rate constant for the unbound drug (kT) across the membrane is the same in both directions. km is the rate constant for binding of drug to the micelles.
Statistical analysis
Statistical analysis was performed using Sigma Stat 3.1 (Systat Software, Inc., Point Richmond, CA). Comparisons between two groups involved the use of the student's t-test with a significance level of p < 0.05. Multiple groups were compared using a one-way analysis of variance, and the Holm-Sidak method was used for the post hoc multiple comparison procedure with a significance level of p < 0.05.
RESULTS
Critical Micellar Concentration
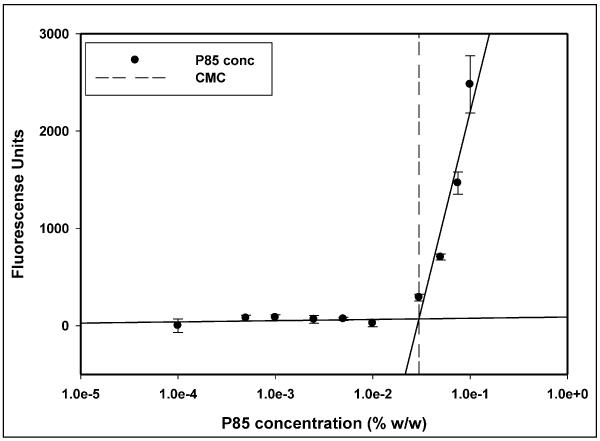
The critical micellar concentration for Pluronic P85 was determined under conditions used in the cellular accumulation assay. In assay buffer at 37°C Pluronic P85 formed micelles at 0.035% w/w (7.5 × 10−5 M) as determined by the DPH fluorescence method (Figure 2).
Figure 2.
Figure 2 is the plot depicting the determination of critical micellar concentration (CMC) of Pluronic P85 in assay buffer at 37°C based on the change in fluorescence intensity of the probe diphenyl hexatriene (DPH) above the CMC. The dashed line represents the CMC of Pluronic P85 determined as the point of intersection of the two solid regression lines, mean ± S.D., n=3).
Dose response studies in MDCK cells
Protease inhibitors
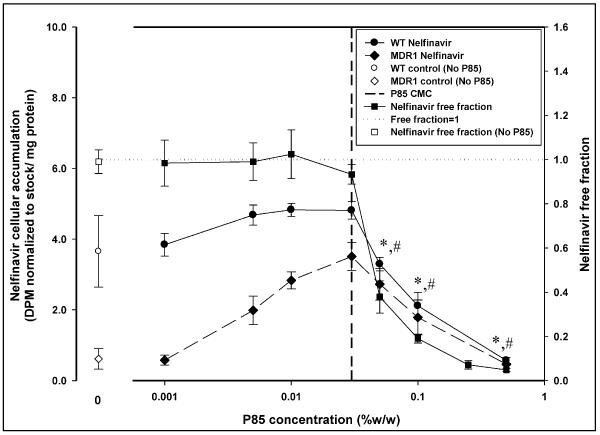
The accumulation of [14C] - nelfinavir in the P-gp over-expressing MDCK-MDR1 cells was significantly lower than in MDCK WT cells in the absence of Pluronic P85. The cellular accumulation of nelfinavir increased significantly in the MDR1 cells as the dose of P85 was increased while there was no statistically significant enhancement in nelfinavir accumulation in the WT cells (Figure 3). The (WT/MDR1) accumulation ratio in the absence of P85 was 6 while at 0.03% w/w P85 this ratio was reduced to 1.4. Above the CMC of Pluronic P85 (0.035% w/w), the accumulation in both the MDR1 cells and the WT cells decreased significantly (Figure 3) and the (WT/MDR1) ratio remained fairly constant. At the highest dose of P85 used (0.5% w/w), the (WT/MDR1) nelfinavir accumulation ratio was 1.23 and accumulation in the MDR1 cells was not statistically different from accumulation in the MDR1 cells in the absence of Pluronic P85 while the accumulation in the WT cells was significantly lower than WT control.
Figure 3.
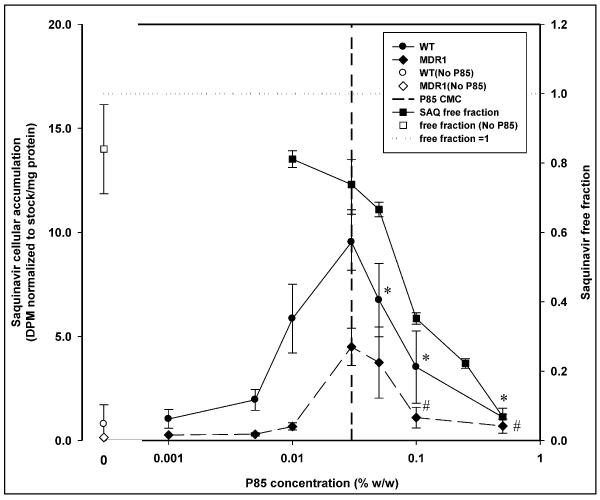
Figure 3 represents the plot of effect of increasing concentration of Pluronic P85 (x-axis, log scale) on nelfinavir cellular accumulation (left y-axis) and nelfinavir free fraction determined by cell-free equilibrium dialysis (right y-axis). The plot shows accumulation of nelfinavir in MDCK WT and MDR1 cells (left y-axis) in the presence (● -WT;◆ -MDR1) and absence (○ -WT;◇ -MDR1) of Pluronic P85; mean ± S.D., n=9. The right y-axis depicts the free fraction of nelfinavir in the presence (∎) and absence (□) of Pluronic P85; mean ± S.D., n=4. The dotted line represents a free fraction of 1 and the vertical dashed line represents the critical micellar concentration of Pluronic P85 (0.03% w/w). *; statistically significant decrease in nelfinavir accumulation in WT cells compared to 0.03% w/w P85 treatment (p<0.05). #; statistically significant decrease in nelfinavir accumulation in MDR1 cells compared to 0.03% w/w P85 treatment (p<0.05).
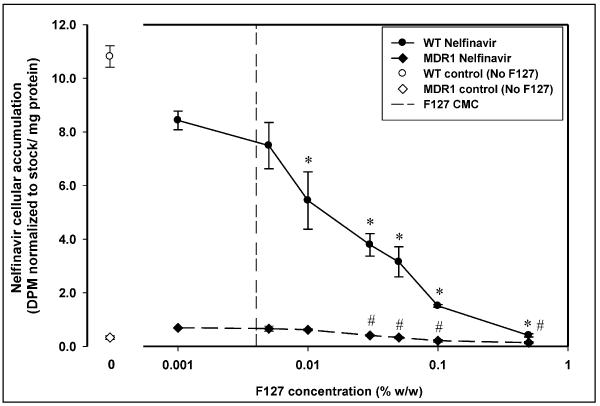
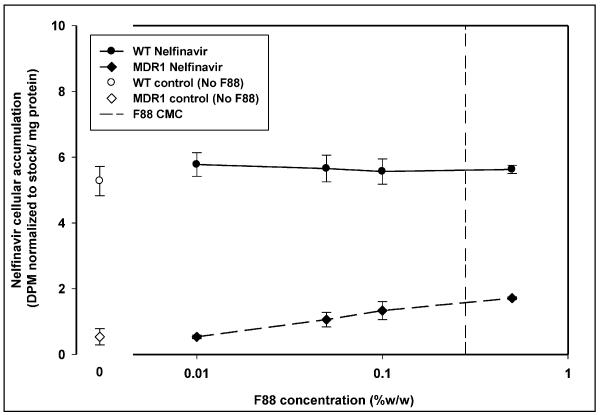
In case of treatment with Pluronic F127 (Figure 4), there was no enhancement in cellular accumulation of nelfinavir at any concentration of F127. The accumulation decreased in both MDCK WT and MDR1 cells with increasing concentration of Pluronic F127 above 0.005% w/w. The (WT/MDR1) accumulation ratio for Pluronic F127 was fairly constant (between 7 and 9) above 0.005% w/w except at the highest concentration of F127 used (0.5% w/w) where it was decreased to 3. Treatment with Pluronic F88 did not result in a decrease in nelfinavir cellular accumulation in the WT or MDR1 cells over the range of concentrations used (Figure 5).
Figure 4.
Figure 4 displays nelfinavir cellular accumulation in the presence of increasing concentration of Pluronic F127 in MDCK WT (●) and MDCK-MDR1 (◆) cells. Cellular accumulation in the absence of Pluronic F127 is shown by open symbols (○ -WT;◇ -MDR1). The vertical dashed line represents the critical micellar concentration of Pluronic F127 (0.004% w/w); mean ± S.D., n=3. *; statistically significant decrease in nelfinavir accumulation in WT cells compared to 0.001% w/w F127 treatment (p<0.05). #; statistically significant decrease in nelfinavir accumulation in MDR1 cells compared to 0.001% w/w F127 treatment (p<0.05).
Figure 5.
Figure 5 depicts nelfinavir cellular accumulation in the presence of increasing concentration of Pluronic F88 in MDCK WT (●) and MDCK-MDR1 (◆) cells. Open symbols depict the cellular accumulation in the absence of Pluronic (○ -WT;◇ -MDR1). The vertical dashed line represents the critical micellar concentration of Pluronic F88 (0.28% w/w); mean ± S.D., n=3.
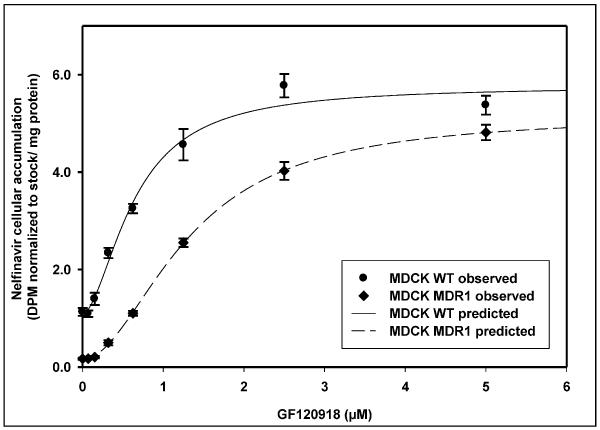
Dose response studies with a small molecule inhibitor of P-gp were conducted in WT and MDR1 cells to compare the effect of a small molecule inhibitor on cellular accumulation of nelfinavir with the results observed from treatment with Pluronic block copolymer. Treatment of MDCK WT and MDR1 cells with increasing concentrations of the small molecule inhibitor GF120918 resulted in a saturable increase in nelfinavir accumulation in both the WT and MDR1 cells. The enhancement in accumulation showed a sigmoidal dose response curve with saturation in response at higher concentrations of GF120918 (Figure 6). There was a 5-fold increase in nelfinavir accumulation in the WT cells at the highest concentration of GF120918 used while the increase in accumulation in the MDR1 cells was 28- fold compared to control (without GF120918). In the WT cells, treatment with GF120918 yielded an Emax of 4.76 ± 0.2 DPM normalized to stock / mg protein (mean ± SE) and an EC50 of 0.64 ± 0.05 μM of GF120918. For the P-gp over-expressing MDR1 cells treated with GF120918 the Emax was 5.01 ± 0.1 DPM normalized to stock / mg protein and an EC50 of 1.32 ± 0.04 μM of GF120918.
Figure 6.
Figure 6 shows the dose response curves for the accumulation of nelfinavir as a function of increasing concentrations of the small molecule P-gp inhibitor GF120918 in MDCK WT (●) and MDCK-MDR1 cells (◆); mean ± S.D., n=3. The sigmoid Emax model with baseline was fit to the data and the fits are represented by the solid line (—) for the WT cells and the dashed line (-----) for the MDR1 cells.
[3H]-Saquinavir showed a significant increase in accumulation in both WT and MDR1 cells (Figure 7) with Pluronic P85 treatment up to the CMC followed by a decline in accumulation at micellar concentrations of P85. Treatment with 0.5% w/w P85 resulted in cellular accumulation in both WT and MDR1 cells that was not significantly different from their respective controls (p<0.05).
Figure 7.
Figure 7 presents the effect of increasing concentration of Pluronic P85 on saquinavir cellular accumulation and saquinavir equilibrium dialysis free fraction. The plot shows accumulation of saquinavir in MDCK WT and MDR1 cells (left y-axis) in the presence (● -WT;◆ -MDR1) and absence (○ -WT;◇ -MDR1) of Pluronic P85; mean ± S.D., n=9. The right y-axis depicts the free fraction of saquinavir in the presence (∎) and absence (□) of Pluronic P85; mean ± S.D., n=4. The dotted line represents a free fraction of 1 and the vertical dashed line represents the critical micellar concentration of Pluronic P85. *; statistically significant decrease in saquinavir accumulation in WT cells compared to 0.03% w/w P85 treatment (p<0.05). #; statistically significant decrease in saquinavir accumulation in MDR1 cells compared to 0.03% w/w P85 treatment (p<0.05).
Vinblastine
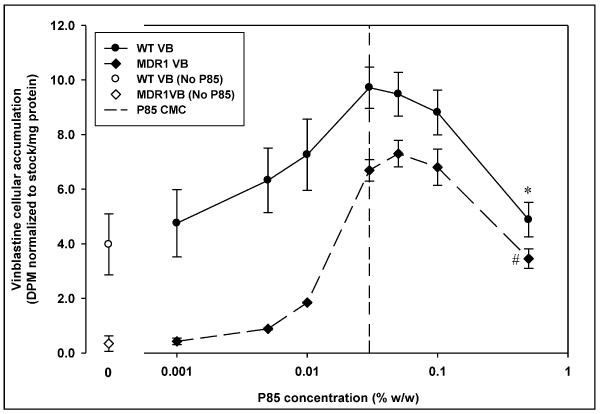
The dose response curve for vinblastine in MDCK cells on treatment with Pluronic P85 showed a different trend from nelfinavir and saquinavir. The decline in vinblastine accumulation above the CMC of Pluronic P85 was significant only at 0.5% w/w, the highest concentration of P85 used (Figure 8). In the WT cells the maximal increase in accumulation was 3-fold at the CMC (0.03% w/w) compared to control and then declined such that the accumulation at 0.5% w/w was 1.5-fold greater than control. In case of the MDR1 cells, the cellular accumulation with P85 treatment increased to a maximum of 21-fold over control at 0.05% w/w of P85 and then decreased to 10-fold over control at P85 concentration of 0.5% w/w (Figure 8).
Figure 8.
Figure 8 represents vinblastine sulfate cellular accumulation in the absence (○ - WT,◇ -MDR1) and presence (● -WT,◆ -MDR1) of incremental concentrations of Pluronic P85 in MDCK WT and MDR1 cells. The vertical dashed line represents the critical micellar concentration of Pluronic P85; mean ± S.D., n=9. *; statistically significant decrease in saquinavir accumulation in WT cells compared to 0.03% w/w P85 treatment (p<0.05). #; statistically significant decrease in saquinavir accumulation in MDR1 cells compared to 0.03% w/w P85 treatment (p<0.05).
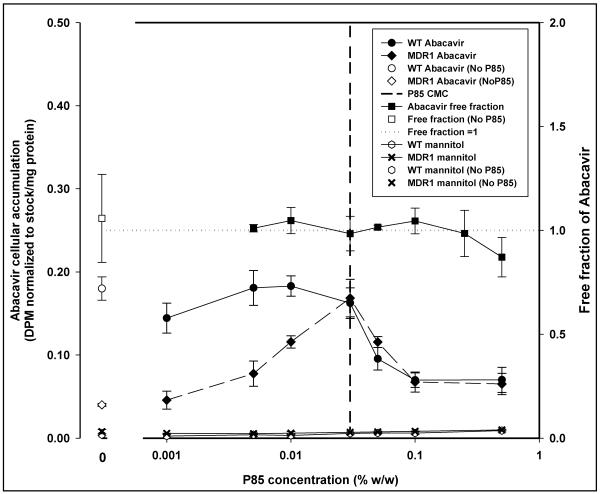
Abacavir
Cellular accumulation of abacavir in MDR1 cells was significantly lower than in WT cells in the absence of Pluronic P85. In the WT cells no significant increase in accumulation occurred up to the CMC and was followed by significant decline in cellular accumulation at all concentrations of P85 above the CMC (p<0.05; Figure 9). In case of MDR1 cells, significant increase in abacavir accumulation compared to MDR1 control was observed up to the CMC followed by a significant decline in accumulation, compared to accumulation at 0.03% w/w, at all concentrations of P85 over the CMC (p<0.05; Figure 9).
Figure 9.
Figure 9 is the change in abacavir cellular accumulation and cell-free equilibrium dialysis free fraction with increasing concentrations of Pluronic P85. The left y-axis depicts cellular accumulation in MDCK WT and MDR1 cells in the presence (● -WT;◆ -MDR1) and absence (○ -WT;◇ -MDR1; mean ± S.D., n=3) of Pluronic P85. Plot also depicts the change in free fraction of abacavir with change in Pluronic P85 concentration (right y-axis) determined experimentally by equilibrium dialysis (∎ -P85,□ - no P85; n=4). *; statistically significant decrease in saquinavir accumulation in WT cells compared to 0.03% w/w P85 treatment (p<0.05). #; statistically significant decrease in saquinavir accumulation in MDR1 cells compared to 0.03% w/w P85 treatment (p<0.05). The effect of Pluronic P85 on mannitol cellular accumulation in MDCK WT and MDR1 cells is also depicted on the plot (▔ -WT; x -MDR1; mean ± S.D., n=9). The horizontal dotted line represents a free fraction of 1 and the vertical dashed line represents the critical micellar concentration of Pluronic P85.
Mannitol
Micellar concentrations of Pluronic P85 had no effect on the accumulation of mannitol (Figure 9). The cellular accumulation of mannitol was low in both MDCK WT and MDR1 cells, with levels in the MDR1 cells being higher than in the WT cells. There was a trend toward increase in accumulation with increasing concentrations of Pluronic P85, which was contrary to what was observed with the P-gp substrates.
Equilibrium dialysis
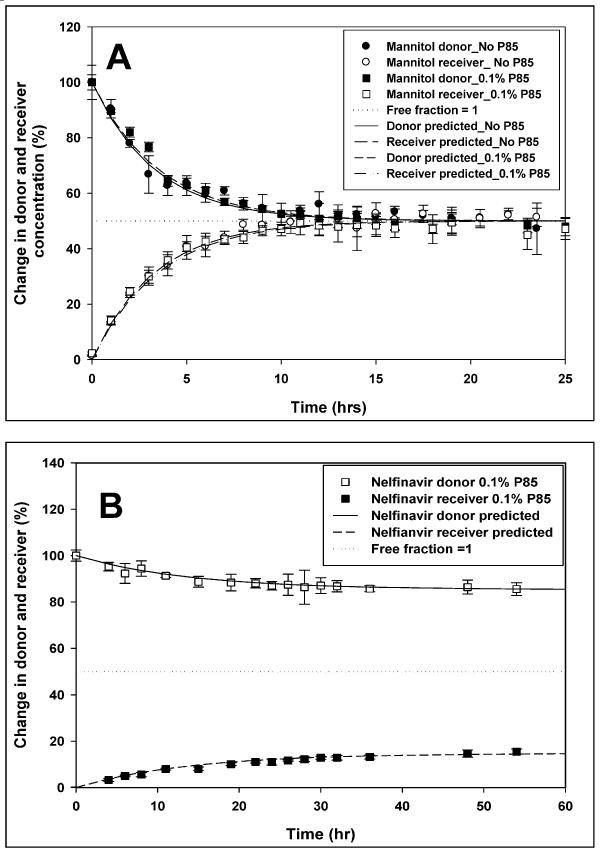
Mannitol
The time to equilibrium for the small hydrophilic molecule mannitol, was ~ 15 hrs and was not affected by micellar concentration (0.1% w/w) of Pluronic P85 (Figure 10a). The equilibrium free fractions of mannitol in the absence and at 0.1% w/w Pluronic P85 were 0.994 ± 0.06 (95% CI: 0.94–1.04) and 0.968 ± 0.04 (95% CI: 0.93–1.01), respectively, and were not significantly different from each other (p>0.05; n=4). The effective permeability of mannitol across the semi-permeable membrane was calculated using equation 5 and was 13.3 ± 1.2 × 10−5 cm/s (mean ± SD) in the absence of Pluronic P85. In the presence of a 0.1% w/w Pluronic P85, the Peff was 12.9 ± 1.6 × 10−5 cm/s and it was not significantly different than the Peff of mannitol in the absence of P85 (p>0.05; n=4).
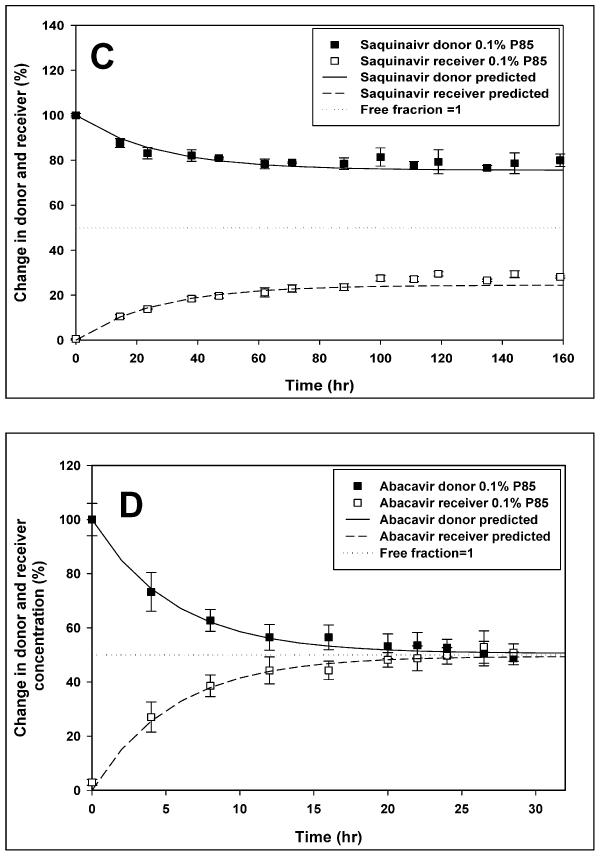
Figure 10.
Figure presents the effect of Pluronic P85 micellar concentration on equilibrium dialysis levels of various drugs. The change in donor and receiver concentrations for (A) Mannitol, (B) Abacavir, (C) Nelfinavir and (D) Saquinavir is depicted as a percentage of donor concentration at time zero with the horizontal dotted line representing a free fraction of 1. All plots represent the equilibrium dialysis of drug at micellar concentration of Pluronic P85 (0.1% w/w;∎ -donor,□ -receiver). Additionally, (A) represents the equilibrium dialysis of mannitol in the absence of Pluronic P85 (● -donor compartment,○ - receiver compartment); mean ± S.D., n=4. The solid line (—) represents the model predicted change in donor and the dashed line (-----) represents the model predicted change in receiver concentration.
Nelfinavir
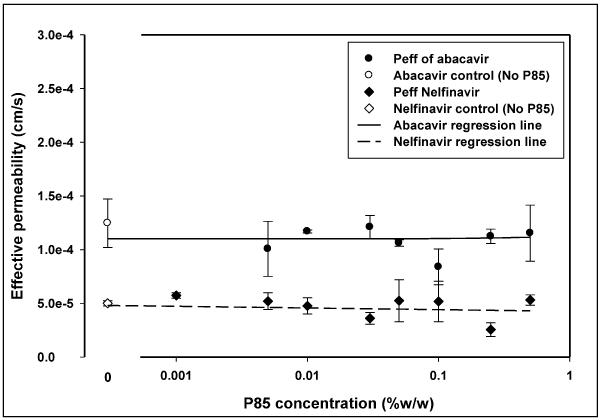
The time to equilibrium for the protease inhibitor nelfinavir, was longer than for mannitol at ~ 50 hrs. The free fraction for the drug in the absence of Pluronic P85 was 0.953 ± 0.109 (95% CI: 0.845–1.060). At all concentrations of Pluronic P85 up to the CMC, the free fraction of nelfinavir was not different from 1 (Figure 3), however at the CMC (0.035% w/w) it was slightly lower than 1 (0.941 ± 0.045; 95% CI: 0.897–0.985) but not statistically different from the free fractions at any of the sub-micellar Pluronic P85 concentrations (p>0.05). Above the CMC of P85 there was a steep decline in the amount of free drug with only 20% of the drug being free at 0.1% w/w (free fraction 0.194 ± 0.02; 95% CI: 0.179–0.209) of Pluronic (Figures 3 and 10c). At 0.5% w/w of P85 only 5% of the drug was free (Figure 3). The presence of Pluronic P85 micelles did not affect the effective permeability of nelfinavir across the membrane. The Peff of nelfinavir was 5 ± 0.25 × 10−5 cm/s in the absence of P85 and 5.31 ± 0.49 × 10−5 cm/s in the presence of 0.5% w/w Pluronic P85 (Figure 11). Simple linear regression of effective permeability of nelfinavir ( Figure 11) on Pluronic P85 concentration yielded a coefficient of determination (R2) value of 0.025 (Figure 11). The affinity constant ka for nelfinavir association with P85 micelles was estimated from the fit of the model (equation 6) to the data and was 73 (%w/w) −1.
Figure 11.
Figure 11 presents the effect of increasing concentration of Pluronic P85 on the effective permeability of nelfinavir and abacavir across the equilibrium dialysis semi-permeable membrane (MWCO 50,000 daltons). Effective permeabilities are shown in the presence (∎-nelfinavir,● -abacavir) and absence (□ -nelfinavir,○ -abacavir) of Pluronic P85; mean ± S.D., n=4. The solid line (—) represents the regression line for abacavir and the dotted line (……) represents the regression line for nelfinavir.
Saquinavir
Saquinavir showed a significant decrease in free fraction with increasing micellar concentrations of Pluronic P85. The free fraction of saquinavir in the absence of Pluronic P85 was 0.827 ± 0.114 (CI: 0.698 – 0.956). At the CMC, the free fraction of drug was measured to be 0.744 ± 0.031 while the free fraction of saquinavir at 0.5% w/w P85 was 0.068 ± 0.007 (Figure 7). There was no effect of Pluronic P85 micelles on the effective permeability of free drug across the membrane (p>0.05). The Peff of saquinavir was 4.7 ± 1.1 × 10−5 cm/s in the absence of P85 and 3.6 ± 0.5 × 10−5 cm/s in the presence of 0.1% w/w Pluronic P85. The affinity constant ka for saquinavir association with P85 micelles was estimated to be 23 (%w/w) −1 (Figure 12).
Figure 12.
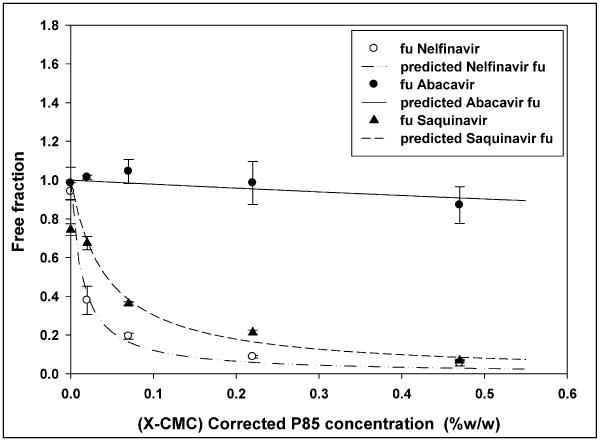
Figure 12 presents the change in free fraction of drug (nelfinavir- ○, abacavir -●, and saquinavir -▲) with change in the micellar concentration of Pluronic P85. The x-axis represents the concentration of P85 micelles, corrected for unimers. The lines represent the model predicted free fraction of nelfinavir (-..-..), abacavir (—) and saquinavir (-----).
Abacavir
The time to equilibrium for the nucleoside reverse transcriptase inhibitor abacavir, was ~ 20 hrs. As seen in figure 9, the free fraction for the drug in the absence of Pluronic P85 was 1.057 ± 0.212 (95% CI: 0.850–1.265). The 95% confidence interval for the free fraction of abacavir at all concentrations of Pluronic P85 bracketed unity except at the highest concentration of 0.5% w/w (free fraction - 0.871 ± 0.095; CI: 0.778 – 0.964). Above the CMC of P85 there was no decline in the free fraction of the drug contrary to what was observed with nelfinavir (Figure 9). The effective permeability of abacavir across the dialysis membrane was not affected by Pluronic and there was no difference in Peff between control 12.5 ± 2.3 × 10−5 cm/s and the highest concentration of P85 (0.5% w/w; 11.5 ± 2.6 × 10−5 cm/s) used (Figure 11). Simple linear regression of effective permeability of abacavir on Pluronic P85 concentration yielded a coefficient of determination (R2) value of 0.0018 (Figure 11). At 0.03 % w/w, the CMC of Pluronic P85, the free fraction of abacavir was 0.984 ± 0.083 (95% CI: 0.903–1.065). At 0.5% w/w P85 roughly 10% of the drug appeared to be associated with the micelles (95% CI: 0.778–0.965). The affinity constant ka of abacavir estimated from the fit of the model to the data was 0.24 (%w/w) −1(Figure 12).
DISCUSSION
HIV-1 protease inhibitor drugs have limited brain penetration in humans resulting in the brain being a sanctuary site for the virus 27. In light of the relatively high lipophilic nature of these drugs, the inability of HIV-1 protease inhibitors to effectively cross the membranes of the blood-brain barrier has been attributed in part, to the active efflux activity of the drug transporter P-glycoprotein 8. Inhibition of P-gp has been reported to improve targeting of HIV-1 protease inhibitors to the brain and a number of small molecule inhibitors of P-gp have been developed and are being tested, especially in the therapy of multi-drug resistant cancers 28,29. Several recent reports have demonstrated the ability of surfactants and block copolymers to inhibit P-gp activity 22,30–32. This is an interesting finding given that these excipients have been considered to be inert and, therefore, devoid of biological activity. Recent reports have also indicated that Pluronics' involvement in altering gene response as well 33,34. We have been investigating the ability of Pluronic block co-polymers to inhibit P-gp-mediated efflux of anti-HIV1 agents, with particular interest in protease inhibitors.
Pluronic P85, one of the Pluronics tested in vitro for their activity against P-gp, was able to effectively inhibit the P-gp-mediated efflux of protease inhibitors, while Pluronics F127 and F88 did not show any effect25. This observation is in agreement with previous reports of Pluronic P85-mediated inhibition of P-gp substrates like rhodamine123 and digoxin in bovine brain microvessel endothelial cells (BBMECs) 16,20. The change in cellular accumulation of P-gp substrates on treatment with Pluronics, as well as with MePEG-b-PCL polymers, has been reported to be a function of polymer concentration 35. A decline in cellular accumulation of P-gp substrates has been reported in P-gp expressing cells exposed to concentrations higher than the CMC of the block copolymers. For the MePEG-b-PCL copolymers, this decline appears to occur at concentrations of the polymer greater than the CMC 15. However, in the case of Pluronics, this decline occur at all concentrations exceeding the CMC 23. In the case of polymers that inhibit P-gp efflux activity (such as P85), this decline in intracellular concentration is a result of micellar trapping, while for polymers devoid of P-gp modulatory activity (such as F127), the effect is simply a decline in intracellular levels of the drug. Therefore, the effect of a range of Pluronic P85 concentrations, both below and above the CMC, on cellular accumulation was determined in P-gp over-expressing MDCKII-MDR1 cells and the MDCKII-WT controls.
The lower accumulation of nelfinavir in MDCK-MDR1 cells compared to the WT cells in the absence of Pluronic P85 (Figure 3), is in accordance with the behavior of a P-gp substrate. The significant 6-fold increase in nelfinavir accumulation when treated with 0.03% w/w P85 when compared to a 1.3-fold increase in WT cells demonstrates P-gp inhibition by Pluronic P85. The modest yet non-significant increase in nelfinavir accumulation in the WT cells is possibly due to inhibition of endogenous P-gp by Pluronic P85 (Figure 3). A similar increase in accumulation below the CMC of P85 was observed for saquinavir (Figure 7), although the increase in accumulation in both the WT and MDR1 cells on Pluronic P85 treatment below the CMC suggests the ability of P85 to inhibit another active transport process present in both cell types. This could be MRP, since it has been reported that MRPs are expressed in MDCKII cells and can transport saquinavir and this transport is inhibited by Pluronic P85 17,36 (see Figure 7).
The above mentioned concentration of 0.03% w/w Pluronic P85 has been reported to be the CMC of Pluronic P85 (average molecular weight 4600; HLB 16) at 37°C and was determined using a pyrene probe method 35. The CMC of Pluronic P85 determined in this study using the fluorescent molecule 1,6-diphenyl-1,3,5- hexatriene (Figure 2) was 0.035% w/w and is in excellent agreement with the CMC determined using the pyrene probe. The decline in drug accumulation in the cell above the CMC for both nelfinavir and saquinavir has many possible explanations. The first possibility is that above the CMC the P-gp inhibitory effect of Pluronic P85 is diminished thereby resulting in a decline in protease inhibitor accumulation due to a resultant increase in active efflux. There are three lines of evidence that do not support this possibility. First, the decline in accumulation above the CMC of Pluronic P85 occurs in both the P-gp over-expressing MDR1 cells, that over-express P-gp, and the WT cells. This observation implies that restoration of P-gp function and increased efflux is unlikely. Secondly, when the effect of Pluronic F127, a non-P-gp inhibiting Pluronic, on nelfinavir accumulation in the same cell system was determined (Figure 4), a similar trend of decrease in cellular accumulation with increasing concentrations of F127 (above the CMC of F127) was observed in both the WT and the MDR1 cells. Also, the accumulation ratios of WT and MDR1 cells for nelfinavir at all concentrations of Pluronic F127 treatment were similar, indicating a similar effect on accumulation in both cell lines that may be independent of the level of P-gp expression (Figure 4). Importantly, the decline in accumulation upon F127 treatment was also above the CMC of F127, indicating that the decline in accumulation is a phenomenon that could be a function of the amphiphilic properties of these Pluronics. Finally, Pluronic P85 mediated inhibition of P-gp has been attributed to the action of the unimers 17,37. The concentration of the unimers increases up to the CMC, and this explains the enhanced cellular accumulation of drug due to P-gp inhibition. Above the CMC, the concentration of unimers remains constant with any further increase in Pluronic resulting in the formation of micelles that are in dynamic equilibrium with the unimers 18. Since the concentration of unimers is constant above the CMC, the ability of Pluronic P85 to inhibit P-gp would not be diminished at concentrations above the CMC.
The effect of the small molecule inhibitor GF120918 on P-gp-mediated efflux of nelfinavir in the same cell system highlights the difference between a small molecule inhibitor of P-gp and block copolymer inhibitors like Pluronic P85 (Figure 6). Treatment with GF120918 resulted in dose response curves that are typical for a saturable inhibition process with both the WT and the MDR1 cells showing a sigmoidal response in nelfinavir accumulation with an approach to a maximum effect as the concentration increases. This provides another line of evidence implicating that Pluronic micelles play a significant role in the decline in accumulation at concentrations of P85 above the CMC.
The decline in cellular accumulation at higher concentrations of polymeric inhibitors of P-gp has been attributed to the association of drug with micelles 5. This is a plausible explanation for the biphasic dose response observed for the cellular accumulation of protease inhibitors. Nerurkar and coworkers used equilibrium dialysis to experimentally determine the decline in free fraction of a model peptide in the presence of micellar concentrations of non-ionic surfactant P-gp inhibitors, and calculated the association constant of the peptide for the surfactants micelles 5. The authors demonstrated the relationship between drug free fraction and the Peff of peptide across a caco2 monolayer and suggested that, above the CMC, micelles could sequester drug thereby decreasing the overall thermodynamic activity of the system. Similarly, Zastre and coworkers reported a decrease in the free fraction of rhodamine123 with increasing concentrations of MePEG-b-PCL block copolymers as measured by equilibrium dialysis, though the decrease in free fraction occurred at concentrations of polymers much greater than the CMC15. Nonetheless, these higher than CMC concentrations still resulted in maximal accumulation of rhodamine 123 in caco-2 cell monolayers. In the case of Pluronics, the decline in accumulation has been reported to occur close to the CMC 16,24. The current study determined the change in free fraction of anti-HIV1 drugs with change in Pluronic P85 concentration using the cell free equilibrium dialysis system and evaluated its relationship to the biphasic cellular accumulation seen in the biological cell based P-gp model system.
Batrakova et al., reported that the enhancement in cellular accumulation of the P-gp substrate digoxin in P-gp over-expressing LLC-PK1 was reversed at P85 concentrations above the CMC16. Digoxin (molecular weight of 780; XlogP of 2.2) is more hydrophilic than protease inhibitors like nelfinavir (molecular weight of 567; XlogP of 6) and saquinavir (molecular weight of 670; XlogP of 3.9), and it would be expected that these hydrophobic drugs could partition into the hydrophobic core of the micelles to a greater extent. This was found to be the case with nelfinavir and saquinavir equilibrium free fractions, 5% and 7% respectively, at 0.5% w/w P85. The association of drug with P85 micelles was calculated using the model proposed by Nerurkar et al.5. The higher lipophilicity of nelfinavir was reflected by its larger association constant for Pluronic P85 micelles when compared to saquinavir (Figure 12).
The lack of decline in accumulation on F88 treatment at concentrations above the CMC (0.28% w/w, Figure 5), could be a function of the composition of the Pluronic. The ability of Pluronic block copolymer micelles to retain drugs has been reported to be a function of the physicochemical properties of the Pluronic such as the hydrophilic-lipophilic balance (HLB), the CMC and the partition coefficient for the drug38. It has been reported that for the hydrophobic probe, pyrene, there exists a linear reciprocal relationship between the partition coefficient of the probe and the Pluronic CMC39. Also, the ability to form stable micelles is related to the relative magnitude of the PPO and PEO blocks 18. Based on the cellular accumulations results for nelfinavir upon treatment with different Pluronics, nelfinavir association with F88 micelles appears to be lower than with F127 and P85 micelles.
Pluronic F88 (average molecular weight 11400) has 80% of its molecular weight composed of the hydrophilic PEO groups, a HLB value of 28, a CMC of 0.28% w/w and the ratio of the hydrophobic PPO region to the hydrophilic PEO region (PPO/PEO) is 0.19. The high CMC, compared to other Pluronics such as F127 (average molecular weight 12600; CMC 0.005% w/w) coupled with the low partition coefficient for hydrophobic pyrene as well as the low (PPO/PEO) ratio could make F88 micelles susceptible to disintegration 39. Thus, the inability of Pluronic F88 to form stable micelles that can trap nelfinavir could explain why the cellular drug accumulation remained unaffected (Figure 5), in contrast to Pluronic F127 which has been classified in the same group of Pluronics as F88 on the basis of HLB number and the magnitude of the PPO block 40. F127 has a HLB of 22 which is lower than for F88 due to a larger PPO block (65 units for F127 vs. 39 units for F88) resulting in a higher (PPO/PEO) ratio of 0.33 and a pyrene partition coefficient of 4.5 (compared to 2.5 for F88). This results in a low CMC of 0.004% w/w 18,39 for F127, leading to the formation of stable micelles with more drug retention capacity than F88. This phenomenon could be reflected in the decrease in cellular accumulation of nelfinavir above the CMC of F127 in the in vitro biological system (Figure 4).
If the change in cellular accumulation following treatment with Pluronic P85 is reflected by a change in the equilibrium free fraction of the drug, then a drug whose free fraction is not affected by micelles would not show any decline in accumulation above the CMC of Pluronic P85. For this reason, both vinblastine and abacavir were selected for their lower lipophilicity compared to protease inhibitors. Results from cellular accumulation and equilibrium dialysis for these drugs indicate that, while there is a relationship between cellular accumulation and micellar trapping, other factors may also be instrumental in determining the effect of block copolymers on the cellular accumulation of drugs.
We examined the effect of Pluronic P85 on vinblastine accumulation to examine the relationship between lipophilicity of drug and micellar trapping in the biological system. The increase in vinblastine sulfate accumulation in both WT and MDR1 cells on P85 treatment may reflect the ability of P85 to inhibit MRP-mediated efflux of vinblastine in both WT and MDR1 cells 41. No effect on the cellular accumulation of vinblastine after 0.03% w/w P85 up to 0.1% w/w (Figure 8) suggests that increase in the number of micelles has no effect on cellular accumulation and unimers are responsible for the increase in accumulation in these cells as has been reported in other studies 5. The decline in accumulation at 0.5% w/w could indicate that vinblastine does not associate with Pluronic micelles to a considerable extent, possibly due to relatively low partitioning (molecular weight of 810; log P 1.89) of vinblastine sulfate 42.
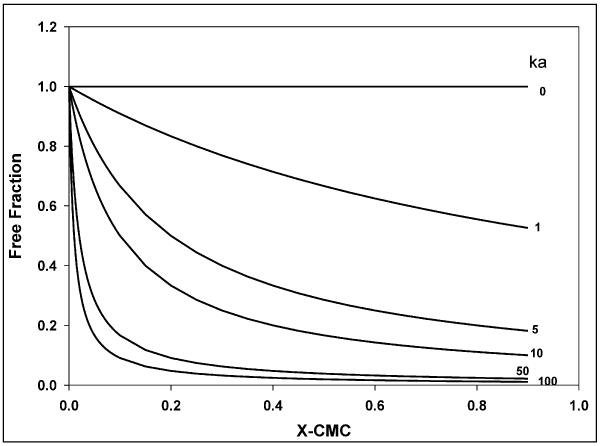
A comprehensive analysis of the relationship between cellular accumulation and free fraction of drug was conducted using the nucleoside reverse transcriptase, abacavir. Abacavir was chosen for its low lipophilicity relative to the protease inhibitors (molecular weight of 286; Xlog P of 1.1), its P-gp substrate status 43, and the fact that abacavir could be a part of combination therapy with a protease inhibitor for the treatment of HIV1. The association constant (ka) for abacavir interaction with P85 micelles was two orders of magnitude lower than for nelfinavir or saquinavir and this was reflected in the free fraction not being different from 1 except at 0.5% w/w P85 (0.871 ± 0.095; Figure 9). Figure 13 depicts the simulated change in free fraction of drug with increasing concentration of micelles and the effect of various ka values on this change. For a given Pluronic, the free fraction is solely a function of and non-linearly related to the ability of the drug to associate with micelles (Equation 6; Figure 13). Thus, abacavir represents a drug that has very low affinity for, and minimal association with, Pluronic P85 micelles. Interestingly, abacavir accumulation did not reflect this result. There was a decrease in accumulation in both WT and MDR1 cells above the CMC of Pluronic P85 (Figure 9), with accumulation in both WT and MDR1 being significantly decline in accumulation above 0.03% w/w P85.
Figure 13.
Figure 13 is a simulation of the effect of change in the affinity rate constant, ka on the free fraction of a drug (—) with increasing Pluronic micellar concentration.
One complicating factor that could be contributing to decline in cellular accumulation is cytotoxicity. It is possible that at higher concentrations Pluronics are toxic to cell monolayers and this cytotoxicity could potentially lead to the decline in cellular accumulation due to loss of cells. It has been reported that Pluronic P85 at a concentration of 0.1% w/w and above can cause cell lysis in caco-2 cells following 90 min of incubation 15. On the other hand, SKVLB and SKVO3 cells exposed to Pluronics P85 up to 1% w/w for 120 min did not show cytotoxicity, though higher Pluronic P85 concentrations and longer incubation times appeared to result in cellular toxicity 44. Yet another study reported that while 5% w/w P85 was not toxic to caco-2 cells it affected cell monolayer confluency in BBMEC cells 21. It is possible that the cytotoxicity of Pluronics varies based on the cell line used. In our studies, the duration of incubation was 180 min and the highest concentration of Pluronics used was 0.5% w/w, though preliminary studies involving the use of up to 1% w/w Pluronic P85 and F88 solutions showed marked toxicity, accessed by changes in the cell protein content as determined by the BCA protein assay and visually under the microscope for gaps in the cell monolayer, following a 180 min accumulation characterized by a loss of cells from the accumulation plate. It must be noted that the concentration of cellular proteins that was determined by the BCA protein assay and used to normalize the cellular accumulation and did not appear to be affected by Pluronics over the range of concentrations used in the studies.
Pluronics are surfactants and can alter the micro-viscosity of the cell membrane rendering it more fluid 40. A recent study by Regev and coworkers, reported that up to 0.1% w/w Pluronic P85 did not affect passive drug permeation across the plasma membrane 32. This is in accordance with the hypothesis that P85 increases the cellular accumulation of drugs by inhibition of P-gp rather than increased membrane permeation. Also, studies with mannitol in both WT and MDR1 cells showed very low levels inside the cells over the entire range of P85 concentrations used (figure 9), implying that Pluronics in either the unimer or micelle form are not causing cell membrane damage, thereby are not enhancing the passive diffusion of mannitol into the cell. It has also been stated that Pluronic micelles are taken up by the cell by endocytosis, probably due to their large size 24. Batrakova et al, have reported that in caco-2 and BBMEC cells, rhodamine 123 uptake at micellar concentrations of Pluronic P85 occurred by fluid phase endocytosis followed by rapid loss of drug from the cells 21. If micelles containing drug are indeed being removed from cells, this phenomenon could explain the decline in accumulation of drugs that associate appreciably with Pluronic micelles but would not explain the decline in abacavir accumulation, since the equilibrium dialysis study for abacavir showed that the drug does not associate with Pluronic P85 micelles.
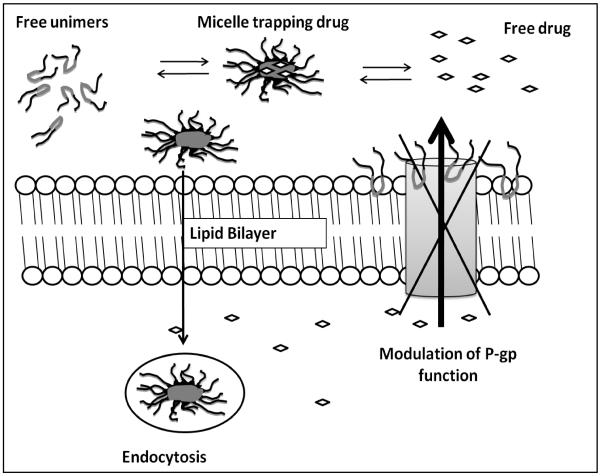
Another possibility, for lipophilic drugs such as nelfinavir, is that as the number of micelles formed increases with increase in the Pluronic concentration above the CMC, a majority of the drug becomes associated with the micelles and the entry of drug into the cells is limited by the rate of endocytosis which would probably be the same at all concentrations of Pluronic used. In such a situation, as the number of micelles formed increases with addition of Pluronic to the solution, more and more drug would be trapped in the micelles and consequently the free fraction of drug available to diffuse into the cells would decrease. If in addition to this the rate of uptake of micelles by the cells via fluid phase endocytosis is unchanged and loss of drug from cells taking up micelles by endocytosis occurs as suggested by Batrakova wt al. 21, then the overall cellular accumulation will decline as the micellar concentration of Pluronic increases (Figure 14). Another interesting question is the effect of unimer-mediated membrane fluidization on the ability of the cell to perform endocytosis. If the change in membrane fluidity would adversely affect fluid phase endocytosis, then the decline in cellular accumulation may be compounded by this phenomenon.
Figure 14.
Figure 14 is a schematic representing the dynamic multiple equilibrium between P85 unimers, self-assembling micelle and free drug. Different physiochemical properties can lead to changes in cellular accumulation.
In summary, the observations made with the protease inhibitors, vinblastine and abacavir regarding the possible relationship between accumulation in the cells and free fraction of the drug available to cross the cell membrane suggests that while there is a definite effect of micellar trapping of drug on the free fraction available to cross membranes, other factors such as cytotoxicity as well as membrane fluidization effects, all of which are independent of P-gp inhibition may also be instrumental in preventing the drug from reaching its target site. Figure 14 describes the various processes that may be at work in a formulation containing drug and Pluronic at micellar concentration. The existence of multiple dynamic equilibria between the unimers, micelles and free drug, P-gp-mediated efflux, inhibition of P-gp by Pluronic, as well as the endocytosis phenomenon would eventually determine the final concentration of anti-HIV1 drugs within the cell. These different processes will determine whether formulation with Pluronics would provide a targeting advantage for drugs by inhibition of P-gp. A well investigated example is that of the anti-cancer drug paclitaxel that is formulated using the non-ionic surfactant Cremophor EL where on the one hand Cremophor EL's ability to inhibit P-gp has been clinically demonstrated 45, but on the other hand it has been implicated in limiting the absorption paclitaxel from the gut in cancer patients, due to micellar trapping 46,47 and also altering the pharmacodynamic characteristics of the drug 48.
In conclusion, this study was able to characterize the biphasic dose response for drug accumulation in the MDCKII-MDR1 cell model on treatment with the novel P-gp inhibitor, Pluronic P85, and use a cell-free equilibrium dialysis system to evaluate the trapping of drug by Pluronic P85 micelles. The model developed for equilibrium dialysis was fit to the experimental data and was used to determine the effective permeabilities as well as to calculate the affinities of drugs for Pluronic micelles. The physicochemical properties of the drug and the composition of the Pluronic will eventually determine the advantage to using a polymer formulation to inhibit P-gp and enhance distribution of drug to a particular site, such as inhibition of P-gp at the blood-brain barrier to increase CNS exposure to anti-HIV1 drugs.
Acknowledgements
This work was supported by National Institutes of Health Grant NS42549. We thank Dr. Piet Borst from the Netherlands Cancer Institute for generously providing the parent MDCKII and P-gp over-expressing MDCKII cells. We would like to thank BASF for generously providing us with Pluronics and to GlaxoSmithKline for their gift of GF120198.
REFERENCES
- 1.Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 2.Gursoy N, Garrigue JS, Razafindratsita A, Lambert G, Benita S. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J Pharm Sci. 2003;92(12):2411–2418. doi: 10.1002/jps.10501. [DOI] [PubMed] [Google Scholar]
- 3.Buckingham LE, Balasubramanian M, Safa AR, Shah H, Komarov P, Emanuele RM, Coon JS. Reversal of multi-drug resistance in vitro by fatty acid-PEG-fatty acid diesters. Int J Cancer. 1996;65(1):74–79. doi: 10.1002/(SICI)1097-0215(19960103)65:1<74::AID-IJC13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock DM, Linsenmeyer ME, Chojnowski G, Kriegler AB, Nink V, Webster LK, Sawyer WH. Reversal of multidrug resistance by surfactants. Br J Cancer. 1992;66(1):62–68. doi: 10.1038/bjc.1992.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nerurkar MM, Burton PS, Borchardt RT. The use of surfactants to enhance the permeability of peptides through Caco-2 cells by inhibition of an apically polarized efflux system. Pharm Res. 1996;13(4):528–534. doi: 10.1023/a:1016033702220. [DOI] [PubMed] [Google Scholar]
- 6.Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y. Effect of excipients on breast cancer resistance protein substrate uptake activity. J Control Release. 2007;124(1–2):1–5. doi: 10.1016/j.jconrel.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001;33(6):475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- 8.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, Kim RB. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28(6):655–660. [PubMed] [Google Scholar]
- 9.Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther. 2003;304(3):1085–1092. doi: 10.1124/jpet.102.045260. [DOI] [PubMed] [Google Scholar]
- 10.Bardelmeijer HA, Ouwehand M, Beijnen JH, Schellens JH, van Tellingen O. Efficacy of novel P-glycoprotein inhibitors to increase the oral uptake of paclitaxel in mice. Invest New Drugs. 2004;22(3):219–229. doi: 10.1023/B:DRUG.0000026248.45084.21. [DOI] [PubMed] [Google Scholar]
- 11.Dordunoo SK, Jackson JK, Arsenault LA, Oktaba AM, Hunter WL, Burt HM. Taxol encapsulation in poly(epsilon-caprolactone) microspheres. Cancer Chemother Pharmacol. 1995;36(4):279–282. doi: 10.1007/BF00689043. [DOI] [PubMed] [Google Scholar]
- 12.Ameller T, Marsaud V, Legrand P, Gref R, Barratt G, Renoir JM. Polyester-poly(ethylene glycol) nanoparticles loaded with the pure antiestrogen RU 58668: physicochemical and opsonization properties. Pharm Res. 2003;20(7):1063–1070. doi: 10.1023/a:1024418524688. [DOI] [PubMed] [Google Scholar]
- 13.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82(2-3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 14.Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surfaces B Biointerfaces. 1999;16:3–27. [Google Scholar]
- 15.Zastre J, Jackson J, Bajwa M, Liggins R, Iqbal F, Burt H. Enhanced cellular accumulation of a P-glycoprotein substrate, rhodamine-123, by Caco-2 cells using low molecular weight methoxypolyethylene glycol-block-polycaprolactone diblock copolymers. Eur J Pharm Biopharm. 2002;54(3):299–309. doi: 10.1016/s0939-6411(02)00119-4. [DOI] [PubMed] [Google Scholar]
- 16.Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296(2):551–557. [PubMed] [Google Scholar]
- 17.Miller DW, Batrakova EV, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16(3):396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- 18.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19(1):1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 19.Batrakova EV, Li S, Li Y, Alakhov VY, Elmquist WF, Kabanov AV. Distribution kinetics of a micelle-forming block copolymer Pluronic P85. J Control Release. 2004;100(3):389–397. doi: 10.1016/j.jconrel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16(9):1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 21.Batrakova EV, Han HY, Miller DW, Kabanov AV. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm Res. 1998;15(10):1525–1532. doi: 10.1023/a:1011942814300. [DOI] [PubMed] [Google Scholar]
- 22.Zastre J, Jackson J, Burt H. Evidence for modulation of P-glycoprotein-mediated efflux by methoxypolyethylene glycol-block-Polycaprolactone amphiphilic diblock copolymers. Pharm Res. 2004;21(8):1489–1497. doi: 10.1023/b:pham.0000036925.45002.a2. [DOI] [PubMed] [Google Scholar]
- 23.Batrakova EV, Han HY, Alakhov V, Miller DW, Kabanov AV. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res. 1998;15(6):850–855. doi: 10.1023/a:1011964213024. [DOI] [PubMed] [Google Scholar]
- 24.Miller DW, Batrakova EV, Waltner TO, Alakhov V, Kabanov AV. Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug Chem. 1997;8(5):649–657. doi: 10.1021/bc970118d. [DOI] [PubMed] [Google Scholar]
- 25.Shaik N, Pan G, Elmquist W. Interactions of pluronic block copolymers on P-gp efflux activity: experience with HIV-1 protease inhibitors. J Pharm Sci. 2008;97(12):5421–5433. doi: 10.1002/jps.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Jackson JK, Burt HM. Determination of surfactant critical micelle concentration by a novel fluorescence depolarization technique. J Biochem Biophys Methods. 1996;31(3–4):145–150. doi: 10.1016/0165-022x(95)00032-m. [DOI] [PubMed] [Google Scholar]
- 27.Kerza-Kwiatecki AP, Amini S. CNS as an HIV-1 reservoir; BBB and drug delivery. J Neurovirol. 1999;5(2):113–114. doi: 10.3109/13550289909021992. [DOI] [PubMed] [Google Scholar]
- 28.Boote DJ, Dennis IF, Twentyman PR, Osborne RJ, Laburte C, Hensel S, Smyth JF, Brampton MH, Bleehen NM. Phase I study of etoposide with SDZ PSC 833 as a modulator of multidrug resistance in patients with cancer. J Clin Oncol. 1996;14(2):610–618. doi: 10.1200/JCO.1996.14.2.610. [DOI] [PubMed] [Google Scholar]
- 29.Planting AS, Sonneveld P, van der Gaast A, Sparreboom A, van der Burg ME, Luyten GP, de Leeuw K, de Boer-Dennert M, Wissel PS, Jewell RC, Paul EM, Purvis NB, Jr., Verweij J. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2005;55(1):91–99. doi: 10.1007/s00280-004-0854-6. [DOI] [PubMed] [Google Scholar]
- 30.Kabanov AV, Batrakova EV, Miller DW. Pluronic block copolymers as modulators of drug efflux transporter activity in the blood-brain barrier. Adv Drug Deliv Rev. 2003;55(1):151–164. doi: 10.1016/s0169-409x(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 31.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm Res. 1999;16(10):1550–1556. doi: 10.1023/a:1015000503629. [DOI] [PubMed] [Google Scholar]
- 32.Regev R, Katzir H, Yeheskely-Hayon D, Eytan GD. Modulation of P-glycoprotein-mediated multidrug resistance by acceleration of passive drug permeation across the plasma membrane. Febs J. 2007;274(23):6204–6214. doi: 10.1111/j.1742-4658.2007.06140.x. [DOI] [PubMed] [Google Scholar]
- 33.Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther. 2006;13(4):804–813. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- 34.Batrakova EV, Kelly DL, Li S, Li Y, Yang Z, Xiao L, Alakhova DY, Sherman S, Alakhov VY, Kabanov AV. Alteration of genomic responses to doxorubicin and prevention of MDR in breast cancer cells by a polymer excipient: pluronic P85. Mol Pharm. 2006;3(2):113–123. doi: 10.1021/mp050050g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res. 1999;16(9):1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 36.Williams GC, Liu A, Knipp G, Sinko PJ. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2) Antimicrob Agents Chemother. 2002;46(11):3456–3462. doi: 10.1128/AAC.46.11.3456-3462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299(2):483–493. [PubMed] [Google Scholar]
- 38.Alakhov VY, Kabanov AV. Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Expert Opin Investig Drugs. 1998;7(9):1453–1473. doi: 10.1517/13543784.7.9.1453. [DOI] [PubMed] [Google Scholar]
- 39.Kozlov MY, Melik-Nubarov NS, Batrakova EV, Kabanov AV. Relationship between Pluronic Block Copolymer Structure, Critical Micellization Concentration and Partitioning Coefficients of Low Molecular Mass Solutes. Macromolecules. 2000;33:3305–3313. [Google Scholar]
- 40.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304(2):845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 41.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm Res. 2003;20(10):1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobert S, Fahy J, Hill BT, Duflos A, Etievant C, Correia JJ. Vinca alkaloid-induced tubulin spiral formation correlates with cytotoxicity in the leukemic L1210 cell line. Biochemistry. 2000;39(39):12053–12062. doi: 10.1021/bi001038r. [DOI] [PubMed] [Google Scholar]
- 43.Shaik N, Giri N, Pan G, Elmquist WF. P-glycoprotein-mediated active efflux of the anti-HIV1 nucleoside abacavir limits cellular accumulation and brain distribution. Drug Metab Dispos. 2007;35(11):2076–2085. doi: 10.1124/dmd.107.017723. [DOI] [PubMed] [Google Scholar]
- 44.Alakhov V, Moskaleva E, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjug Chem. 1996;7(2):209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 45.Webster L, Linsenmeyer M, Millward M, Morton C, Bishop J, Woodcock D. Measurement of cremophor EL following taxol: plasma levels sufficient to reverse drug exclusion mediated by the multidrug-resistant phenotype. J Natl Cancer Inst. 1993;85(20):1685–1690. doi: 10.1093/jnci/85.20.1685. [DOI] [PubMed] [Google Scholar]
- 46.Bardelmeijer HA, Ouwehand M, Malingre MM, Schellens JH, Beijnen JH, van Tellingen O. Entrapment by Cremophor EL decreases the absorption of paclitaxel from the gut. Cancer Chemother Pharmacol. 2002;49(2):119–125. doi: 10.1007/s00280-001-0394-2. [DOI] [PubMed] [Google Scholar]
- 47.Malingre MM, Schellens JH, Van Tellingen O, Ouwehand M, Bardelmeijer HA, Rosing H, Koopman FJ, Schot ME, Ten Bokkel Huinink WW, Beijnen JH. The co-solvent Cremophor EL limits absorption of orally administered paclitaxel in cancer patients. Br J Cancer. 2001;85(10):1472–1477. doi: 10.1054/bjoc.2001.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles : implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42(7):665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]