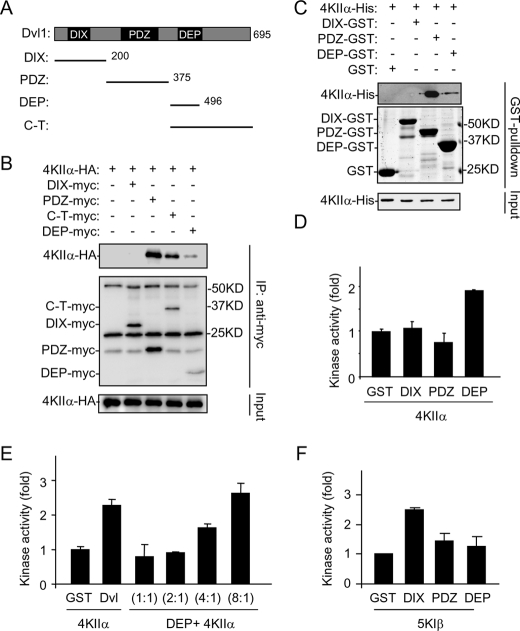
FIGURE 4.
Characterization of Dvl domains in interaction and activation of PI4KIIα. A, schematic representation of Dvl1 and its truncated forms used in the study. B, delineation of Dvl1 domains involved in interaction with PI4KIIα by immunoprecipitation with proteins transiently expressed in HEK293T cells. Three background bands existing in all of the lanes are Ig. C, in vitro pull-down assay using recombinant Dvl1 fragments and PI4KIIα. Samples were analyzed by immunoblotting with the indicated antibodies or Coomassie Blue staining. D and E, effects of Dvl1 fragments on lipid kinase activity of PI4KIIα. Purified PI4KIIα (50 nm) was assayed for its lipid kinase activity in the presence of 200 nm (D) or varying amounts of recombinant proteins (E). The ratio indicates the amount of DEP domain protein to PI4KIIα protein. The amounts of GST and Dvl used in E are 50 nm. The data are presented as means ± S.E. F, effects of Dvl1 fragments on lipid kinase activity of PIP5KIβ. Purified PIP5KIβ (50 nm) was assayed for its lipid kinase activity in the presence of 50 nm Dvl domain proteins.