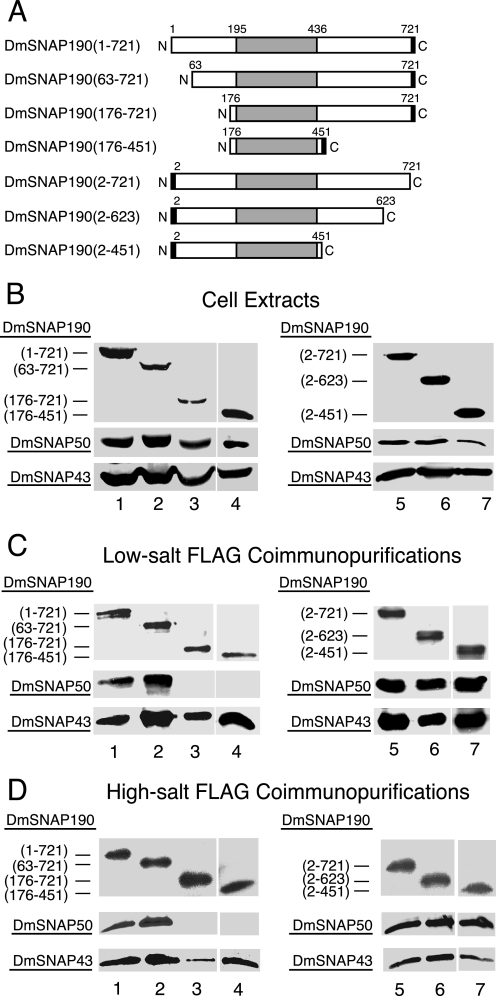
FIGURE 2.
Domains of DmSNAP190 involved in assembly in vivo with DmSNAP50 and DmSNAP43. A, schematic representation of full-length and truncated DmSNAP190 constructs co-transfected into S2 cells together with constructs expressing full-length untagged DmSNAP50 and DmSNAP43. The shaded area represents the 4.5-Myb-repeat domain conserved between humans and fruit flies. The black rectangles represent FLAG tags at the C or N termini of DmSNAP190. The names of the constructs in the column at the left indicate the extent of the wild type amino acid residues present in the expressed constructs. B, co-overexpression of tagged DmSNAP190 with DmSNAP50 and DmSNAP43 in stably transfected S2 cells. Whole cell extracts from stably transfected cells co-overexpressing all three DmSNAP subunits were run on denaturing gels and DmSNAP subunits were detected by immunoblot analysis. The amount of extract loaded in each lane was normalized so that the intensity of the signal obtained from the tagged DmSNAP190 construct was similar in each lane. The top panels show full-length or truncated tagged DmSNAP190 detected using monoclonal antibodies against the FLAG epitope. The middle and bottom panels show detection of DmSNAP50 or DmSNAP43, respectively, by using polyclonal anti-peptide antibodies prepared against C-terminal peptides of the respective proteins (14). For all immunoblots shown in this and subsequent figures, the lanes in each horizontal panel (top, middle, or bottom) are from the same gel blot developed for the same length of time. Thus the intensities of the bands within a horizontal panel reflect the relative amounts of protein present in the various transfected cell lines. C, co-purification of DmSNAP50 and DmSNAP43 with full-length and truncated DmSNAP190 constructs following FLAG affinity purification under low-salt conditions. Complexes containing full-length or truncated tagged DmSNAP190 were purified using FLAG antibody beads, and the presence of the individual subunits in the elution fractions was evaluated by immunoblotting. The volume of elution fraction loaded in each lane was normalized so that the intensity of the signal for the DmSNAP190 construct was similar in each lane when detected with FLAG antibodies. DmSNAP50 and DmSNAP43 subunits that co-purified with the FLAG-tagged DmSNAP190 constructs were detected with antibodies prepared against C-terminal peptides of the respective proteins. D, same as C except FLAG affinity purification was carried out under high-salt conditions (0.35 m NaCl).