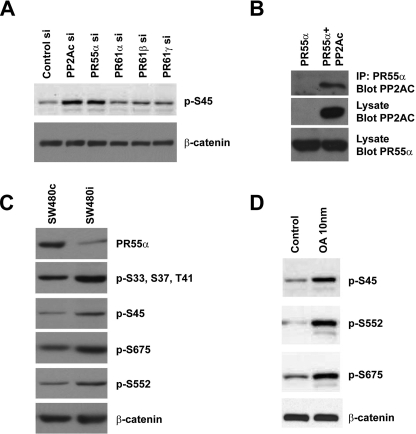
FIGURE 3.
Identification of PR55α as the regulatory B subunit that controls β-catenin phosphorylation. A, SW480 cells were transfected with siRNAs targeting several different regulatory subunits of PP2A. Negative control siRNA and PP2Ac siRNA were used as controls. Total β-catenin and phosphorylated β-catenin were analyzed by Western blot with anti-Ser(P)-45 and anti-β-catenin Abs. B, interaction between PR55α and PP2Ac. Myc-tagged PR55α was cotransfected with CS2 control plasmid or FLAG-tagged PP2Ac into HEK293T cells. PR55α protein was immunoprecipitated from cell lysates with an anti-Myc Ab. The presence of PP2Ac in the immunoprecipitated (IP) samples was analyzed by Western blot with an anti-FLAG Ab. The levels of PP2Ac and PR55α in the cell lysates were analyzed as control. C, SW480 cells were infected with lentiviruses that express control shRNA or PR55α shRNA. Stable cells were selected with puromycin. PR55α protein levels in these cells were analyzed by Western blot with an anti-PR55α Ab. Total β-catenin and phosphorylated β-catenin were analyzed with an anti-β-catenin Ab and phospho-specific Abs that recognize different phosphorylation sites of β-catenin. D, SW480 cells were treated with dimethyl sulfoxide or 10 nm OA. Total β-catenin and phosphorylated β-catenin were analyzed with an anti-β-catenin Ab and phospho-specific Abs against β-catenin.