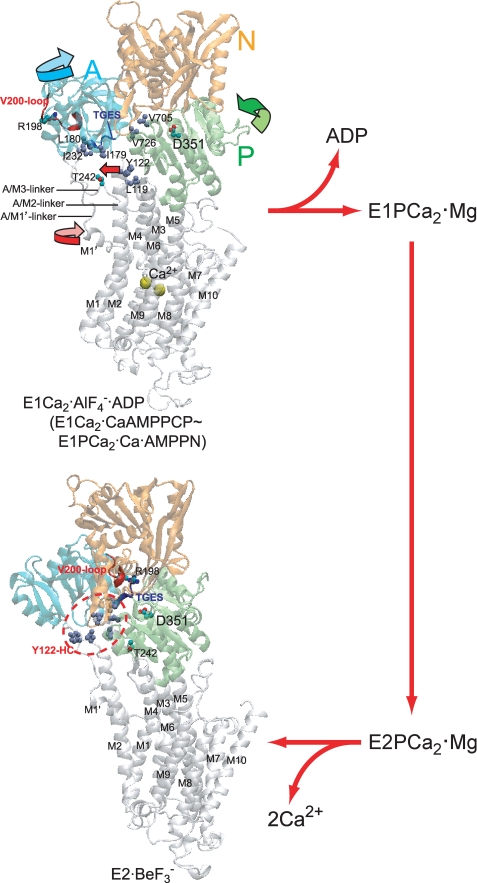
FIGURE 2.
Structure of SERCA1a and its change during processing of phosphorylated intermediate. E1Ca2·AlF4−·ADP (the transition state analog for phosphorylation E1PCa2·ADP·Mg‡) and E2·BeF3− (the ground state E2P analog (25)) were obtained from the Protein Data Bank (PDB accession code 1T5T (17) and 2ZBE (21), respectively). Cytoplasmic domains N (nucleotide binding), P (phosphorylation), and A (actuator), and 10 transmembrane helices (M1–M10) are indicated. The arrows on the domains, M1′ and M2 (Tyr122) in E1Ca2·AlF4−·ADP, indicate their approximate motions predicted for E1PCa2·ADP·Mg‡ → E2P·Mg. The phosphorylation site Asp351, TGES184 of the A domain, Arg198 (tryptic T2 site) on the Val200 loop (DPR198AV200NQD) of the A domain, and Thr242 (proteinase K site) on the A/M3-linker are shown. Seven hydrophobic residues gather in the E2P state to form the Tyr122-hydrophobic cluster (Y122-HC); Tyr122/Leu119 on the top part of M2, Ile179/Leu180/Ile232 of the A domain, and Val705/Val726 of the P domain. The overall structure of E1Ca2·AlF4−·ADP is virtually the same as those of E1Ca2·CaAMP-PCP and E1PCa2·Ca·AMP-PN (17, 18, 22).