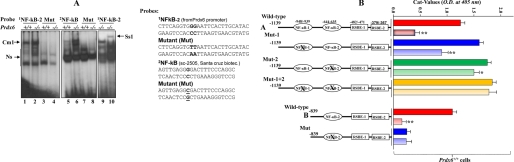
FIGURE 7.
A, nuclear extract from Prdx6−/− cells strongly bound to the NF-κB-responsive element present in the Prdx6 promoter. The 32P-labeled oligonucleotide probes 1NF-κB-2 and 2NF-κB (as standard control) and their mutants were incubated with nuclear extract isolated from Prdx6−/− and Prdx6+/+ cells, and a gel shift assay was performed. A supershift assay using Rel/P65 antibody following incubation with Prdx6−/− nuclear protein extract was carried out (Ss1; lane 10). B, point mutation at NF-κB sites identified by gel shift assay experiment (NF-κB-2) in the Prdx6 promoter Construct B abolished its transcriptional activity, whereas disruption of NF-κB-1 and/or NF-κB-2 in Construct A increased the promoter activity. Prdx6+/+ or Prdx6−/− cells were transiently transfected with equal amounts of wild type and their mutant promoter-driven CAT reporters (left panel, showing schematic representation of constructs in the experiment), and the CAT activity was measured. For Construct A, CAT activity of promoter mutated at NF-κB sites (Mut-1 (blue bar), Mut-2 (green bar), or Mut1 + Mut-2 (yellow bar)) was compared with the activity of wild type promoter constructs (WT (red bar)). In the same set of experiments, cells were also cotransfected with dominant-negative Iκ-Bα mutant (Iκ-BαAA; light red bar, light blue bar, and light yellow bar). For Construct B, cells were transfected either with wild-type NF-κB or its mutant, and CAT activity was monitored (B, WT versus Mut). In another set of experiments, cells were cotransfected with Iκ-BαAA (Construct B, light red and light blue bars). The CAT vector showed insignificant activity (data not shown).