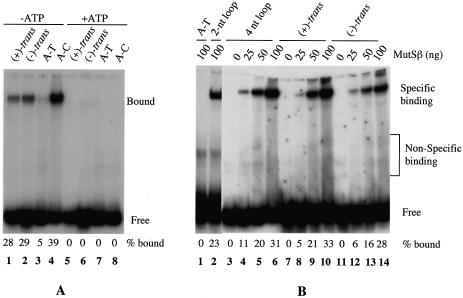
Figure 2.
Binding of MutSα and MutSβ to DNA containing B[c]PhDE-dA adducts. Unless otherwise indicated, gel-shift assays were performed in 25 µl reactions containing 0.5 pmol 32P-labeled oligonucleotide duplexes, MutSα (100 ng) or MutSβ (25–100 ng). After 20 min incubation on ice, 5 µl 50% sucrose was added. The samples were fractionated at room temperature through a 6% non-denaturing polyacrylamide gel in 6.7 mM Tris–acetate (pH 7.5) and 1 mM EDTA with buffer recirculation. When present, ATP is at a final concentration of 1 mM. (A) Interaction of MutSα with B[c]PhDE-dA substrates. (B) Interaction of MutSβ with B[c]PhDE-dA substrates. (+)-trans, (+)-trans-anti-B[c]PhDE; (–)-trans, (–)-trans-anti-B[c]PhDE; A-T, oligonucleotide substrate containing no mismatches; A-C, oligonucleotide substrate containing an A-C mismatch; 2-nt loop, oligonucleotide substrate containing two unpaired (or ID) nucleotides; 4-nt loop, oligonucleotide substrate containing four unpaired nucleotides.