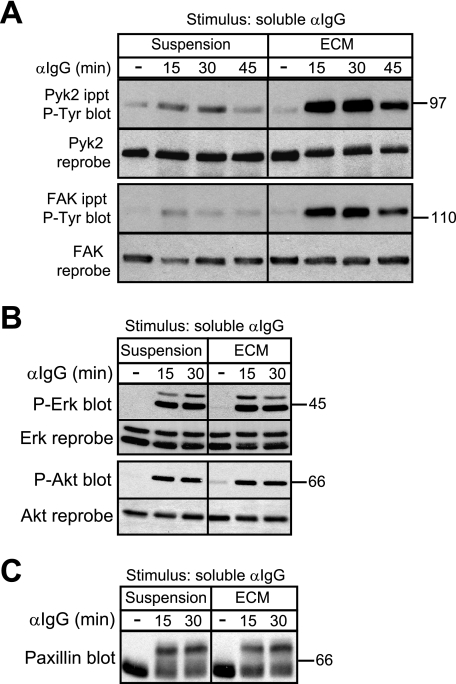
FIGURE 2.
Adhesion of B cells to ECM selectively enhances BCR-induced tyrosine phosphorylation of Pyk2 and FAK. A20 cells were kept in suspension or plated on collagen/fibronectin ECM for 30 min before being stimulated with 20 μg/ml soluble anti-IgG for the indicated times. For unstimulated controls (−), A20 cells were kept in suspension or plated on collagen/fibronectin ECM for 30 min and then left unstimulated for an additional 45 min before being lysed. A, immunoprecipitated (ippt) Pyk2 and FAK were analyzed by immunoblotting with the 4G10 anti-Tyr(P) (P-Tyr) Ab. The blots were then reprobed with Abs to Pyk2 or FAK. A mock stimulation of cells with phosphate-buffered saline for 15 or 30 min did not increase phosphorylation of Pyk2 and FAK compared with cells left unstimulated for the entire duration of the experiment (supplemental Fig. 2). B, cell lysates were immunoblotted with Abs against the phosphorylated forms of Erk (P-Erk) or Akt (P-Akt) and then reprobed with Abs against total Erk or Akt. C, cell lysates were immunoblotted with a paxillin Ab. Serine/threonine phosphorylation of paxillin is indicated by a bandshift on SDS-PAGE gels and was dependent on the activity of the Erk and GSK-3 kinases (data not shown) as in T cells and macrophages (67, 68). For each panel, similar results were obtained in three experiments. αIgG, anti-IgG Ab.