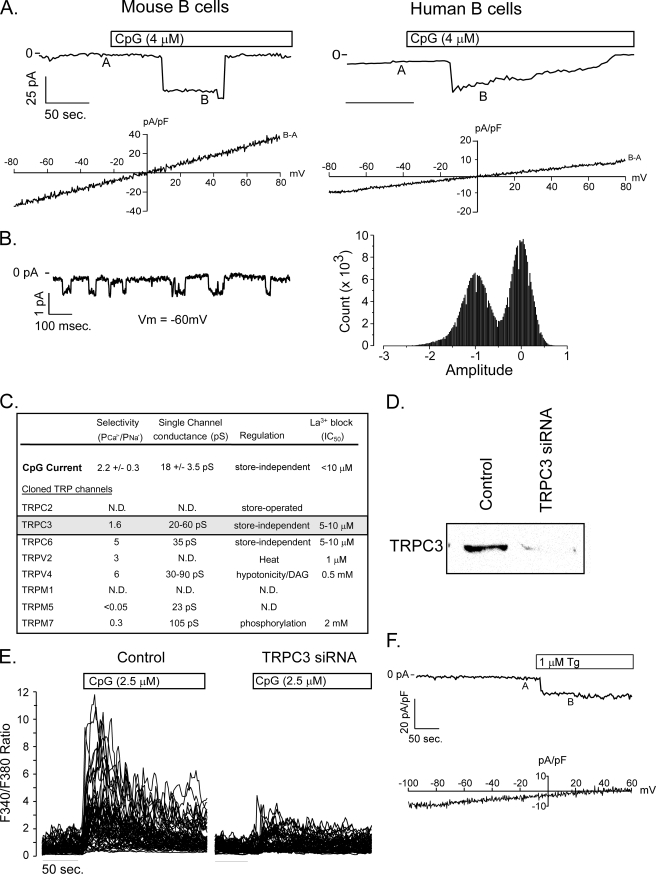
FIGURE 2.
CpG induced calcium entry is mediated by TRPC3. A, patch clamp recording of steady-state current elicited by CpG in primary mouse (left) and human (right) B cells at −70 mV holding potential. Voltage ramps (−80 mV to +80 mV) recorded at A were subtracted from ramps applied at B, and the I-V relationship was plotted beneath each macroscopic current. The current reversal potential of ∼0 mV in both cell types indicates that CpG activates a non-selective cation channel in primary B cells. These currents are representative of measurements obtained from more than 25 mouse and 3 of 8 human primary B cells. B, single channel activity of CpG-induced channel at a holding voltage of −60 mV (left) and current amplitude frequency histogram (right). C, the calculated single channel conductance, relative Ca2+ versus Na+ permeability, and store dependence of activation for CpG currents in B lymphocytes and published values for TRP family members (53, 54) expressed by primary murine B cells (5) indicate that CpG-induced currents most closely resemble TRPC3. D, Western analysis demonstrates almost complete suppression of TRPC3 protein expression in primary murine B lymphocytes. Lysates were obtained from 5 million primary murine B lymphocytes 48 h after transfection with TRPC3 siRNA. E, TRPC3 siRNA transfection did not affect BCR-induced Ca2+ signals, but almost completely suppressed CpG-induced responses. E, thapsigargin elicits an inward current in primary B cells (upper panel). The background-subtracted I-V current (B − A, bottom panel) exhibits inward rectification consistent with CRAC and not non-selective cation channel activation by thapsigargin.