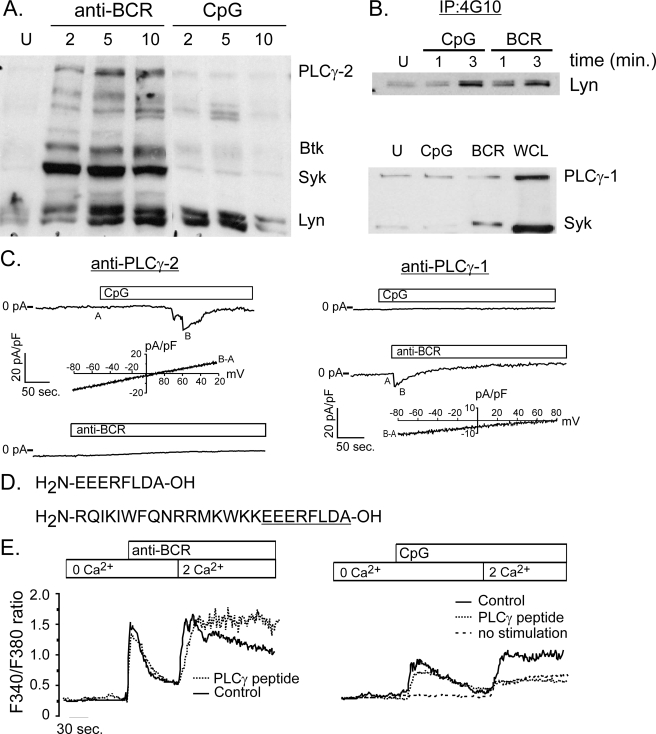
FIGURE 3.
CpG-induced calcium signals are mediated by PLCγ-1. A, phosphotyrosine (4G10) Western analysis of protein lysates from BCR- and CpG-activated primary murine B cells. BCR engagement induces phosphorylation of key enzymes, including Lyn, Syk, Btk, and PLCγ-2. By contrast, CpG stimulation does not activate Syk, Btk, or PLCγ-2, but does trigger a transient increase in tyrosine phosphorylation of a protein with mobility identical to Lyn kinase. B, Western blot analysis of phosphotyrosine immunoprecipitates confirms Lyn phosphorylation by CpG at levels comparable to that induced by BCR stimulation (upper panel). Although PLCγ-1 is expressed in primary B cells, neither CpG nor BCR engagement triggers a detectable increase in its phosphorylation (bottom panel). An increase in phospho-Syk levels following BCR engagement indicates that cells are activated by this stimulus. C, intracellular dialysis of anti-PLCγ-1 antibody (right) but not PLCγ-2 (left) blocks CpG activation of TRPC3 currents in primary B cells. By contrast, anti-PLCγ-2, but not anti-PLCγ-1 blocks CRAC current activation by anti-BCR antibody. I-V plots below CpG and CRAC macroscopic currents are shown to demonstrate identity of respective currents. D, sequence is shown for peptide that represents the domain within PLCγ-1 that interacts with TRPC3 channels. Also shown (bottom) is the same PLCγ-1 peptide constructed with an N-terminal antennapedia sequence (underlined) to render the peptide membrane permeant (55). E, the membrane-permeant PLCγ-1/TRPC3 peptide was used to examine the role of TRPC3 in BCR- and CpG-induced Ca2+ entry in primary B cells. The peptide did not inhibit BCR-induced Ca2+ release from stores or subsequent Ca2+entry into cells (left). By contrast, this PLCγ-1/TRPC3 interacting domain peptide completely blocked CpG-induced Ca2+ entry (right). The residual Ca2+ entry observed in the presence of peptide was also seen in unstimulated cells following switch from Ca2+ free to Ca2+ containing external solution (dotted line).