FIGURE 1.
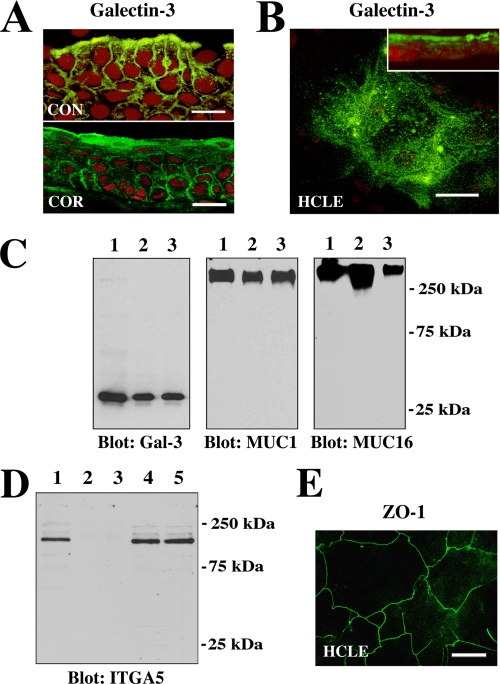
Galectin-3 localizes at the apical surface of normal epithelia in vivo and in vitro. A, confocal immunofluorescence micrograph showing binding of the rat anti-galectin-3 antibody M3/38 (green) to apical membranes of apical epithelial cells in human conjunctival (CON) and corneal (COR) tissue cross-sections. Use of the rabbit anti-galectin-3 antibody H-160 resulted in a similar staining pattern (data not shown). Propidium iodide (red) was used to visualize the cell nuclei. Magnification bars = 20 μm. B, in HCLE cells, binding of the galectin-3 antibody was predominantly apical, as evidenced along the x-y axis (en face view) and in cell culture cross-sections (inset). Magnification bar = 20 μm. C, cell surface proteins on apical cell membranes of HCLE cells were biotinylated, purified through a neutravidin-agarose affinity column, and immunoblotted with anti-galectin-3, anti-MUC1, and anti-MUC16 antibodies. Control lanes were loaded with 15 μg of total protein from HCLE cell lysates (lane 1). Biotinylation experiments were performed in duplicate, and apical proteins were detected in the bound fraction eluted from the affinity column (lanes 2 and 3). D, to determine whether biotinylation was restricted to the apical cell surface, membrane extracts of labeled cells were analyzed for the presence of integrin α5 subunit (ITGA5), a basolateral cell membrane glycoprotein in corneal epithelium (19). In these experiments ITGA5 was not detected in the bound fraction eluted from the neutravidin affinity column (lanes 2 and 3) but was detected in the unfractionated extract (lane 1) as well as in the flow-through fractions (lanes 4 and 5). E, tight junctions in HCLE cells stained with the anti-ZO-1 antibody. The image was acquired along the x-y axis and corresponds to the distribution of tight junctions observed in normal corneal epithelial cells (24). Magnification bar = 50 μm.