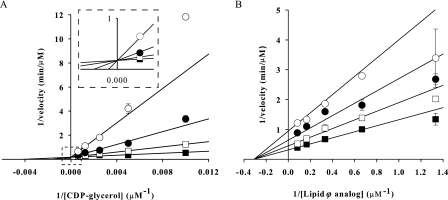
FIGURE 4.
Inhibition of TagF by CMP. Inhibition of TagF by the reaction product CMP is presented in double reciprocal plots of 1/velocity versus 1/[substrate]. A, CMP was held constant at 0 (■), 300 (□), 1000 (●), and 3000 (○) μm while CDP-glycerol was varied between 100 and 1600 μm, and Lipid ϕ analog was fixed at 2 μm. B, CMP was held constant at 0 (■), 100 (□), 300 (●), and 1000 (○) μm while the Lipid ϕ analog was varied from 1 to 16 μm with CDP-glycerol fixed at 180 μm. Experiments were conducted with 2.5 nm TagF, and initial rates were determined by monitoring the conversion of [α-32P]CDP-glycerol to [α-32P]CMP via PIC-HPLC. Rate data were fit to all inhibition models in the Enzyme Kinetics Module 1.1 for Sigma Plot 8.0 using a non-linear sum of least squares regression method. When CDP-glycerol was the varied substrate the data were best described by the competitive inhibition model (Equation 3). With the Lipid ϕ analog as the varied substrate the data were best described by the non-competitive (partial) inhibition model (Equation 4). Error bars indicate S.E.