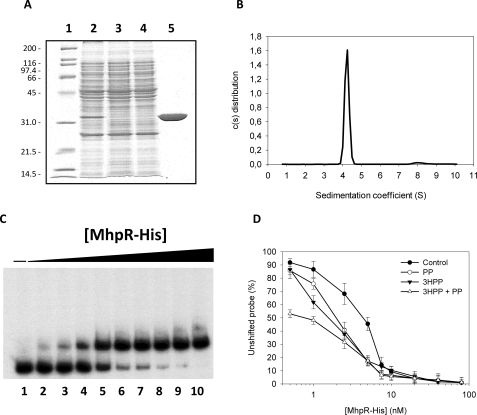
FIGURE 4.
A, overexpression and purification of MhpR-His protein. Analysis on a 12.5% SDS-PAGE of the purification of MhpR-His from E. coli M15 (pREP4, pQMH) cells. Lane 1, molecular mass markers shown in kDa; lane 2, soluble fraction of the crude extract from E. coli M15 (pREP4, pQMH); lane 3, extract that flows through the nickel-nitrilotriacetic acid-agarose column; lane 4, washing step; lane 5, purified MhpR-His protein loaded at 1.5 mg/ml. B, distribution of the sedimentation coefficients for the MhpR-His protein. c(s) sedimentation coefficient distribution was at a protein concentration of 0.5 mg/ml, a rotor temperature of 20 °C, and a rotor speed 200,000 × g. C, gel retardation analyses of MhpR-His binding to the Pa-Pr promoter in absence of aromatic compound. Increasing concentrations of purified MhpR-His were used: lane 1 (0 nm), lane 2 (0.5 nm), lane 3 (1 nm), lane 4 (2.5 nm), lane 5 (5 nm), lane 6 (7.5 nm), lane 7 (10 nm), lane 8 (20 nm), lane 9 (40 nm), and lane 10 (80 nm). Pa-Pr concentration was 1 nm. D, determination of the Kdfor MhpR-His binding to the mhpR-mhpA intergenic region in the absence (closed circles) and presence of 0.5 mm PP (open circles), 0.5 mm 3HPP (closed triangles), or at 0.25 mm both compound (open triangles). The Kd was the MhpR-His concentration at which 50% of the total probe was bound. This value was determined from the curves. The concentration of the probe is significantly lower than the MhpR-His concentration. Errors bars are S.D.