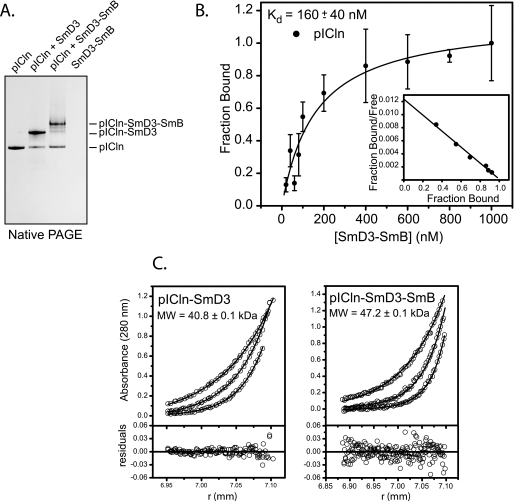
FIGURE 2.
Interaction of pICln with Sm proteins. A, native PAGE of purified pICln, pICln·SmD3 complex, and a 2:1 mixture of pICln with SmD3/SmBΔC heterodimer. The SmD3/SmBΔC dimer is positively charged and, therefore, does not enter the polyacrylamide gel unless it is bound to pICln. B, pICln binding to SmD3/SmBΔC monitored by fluorescence polarization of labeled pICln. pICln binds with a Kd of 160 nm and a 1:1 stoichiometry. Error bars represent 1 S.D. derived from three replicate experiments. C, sedimentation equilibrium ultracentrifugation of pICln·SmD3 complex at 25 μm and pICln·SmD3/SmBΔC complex at 12.5 μm concentrations. pICln·SmD3 data fit well to a 1:1 heterodimer, and pICln·SmD3/SmBDC data fit well to a 1:1:1 heterotrimer with the experimental molecular weights shown.