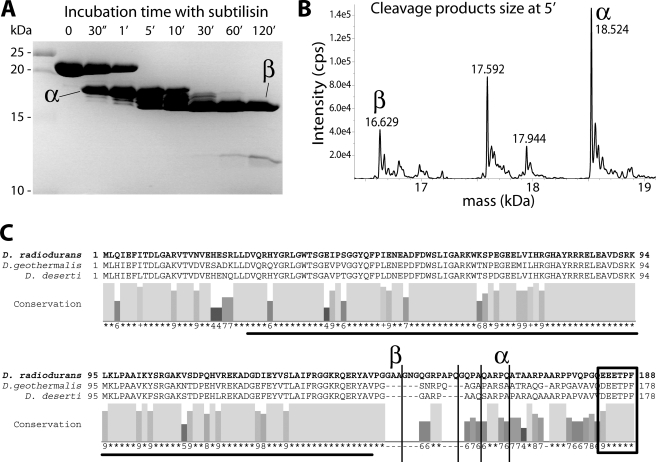
FIGURE 4.
Limited proteolysis produces cleavage within the predicted unstructured domain, between the putative OB-fold and the C terminus motif similar to that used by SSB proteins to interact with partners. A, incubation of DdrB protein (48 μm) at room temperature with a low concentration of subtilisin (74 nm) shows the appearance over time of two main cleavage products: α, and later a species we label β that proves more resistant to the protease. B, mass spectrometry size determination, using ESI-Qtrap, allowed the identification of the exact corresponding DdrB subsequences for the products present after 5 min of incubation. C, cleavage sites (vertical lines) reported on D. radiodurans DdrB sequence (bold), aligned with DdrBs from D. geothermalis and D. deserti, showing their occurrence in the unconserved region that corresponds to a putative flexible arm between the potential OB-fold domain (underlined) and the C terminus motif (box).