FIGURE 5.
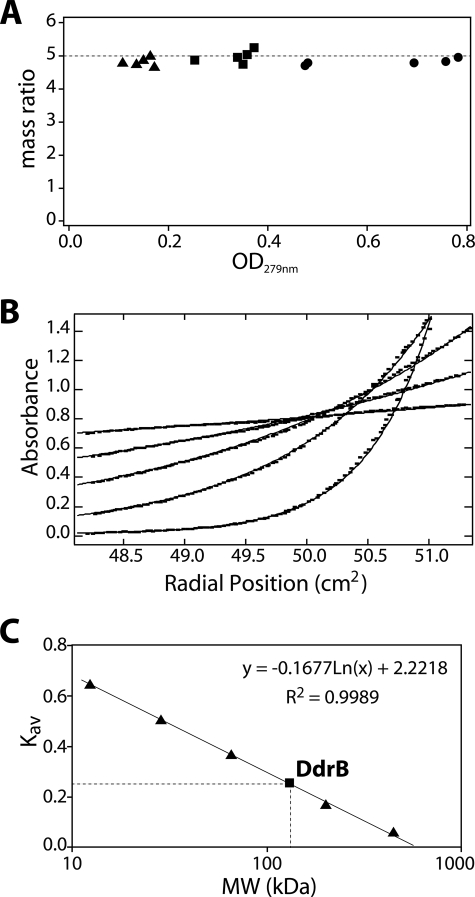
Native DdrB is a pentamer in solution. A, sedimentation equilibrium performed using analytical ultracentrifugation for three DdrB concentrations (▴, 5.85 μm; ■, 11.99 μm; and ●, 24.12 μm) at five speeds (3,600, 6,300, 8,700, 12,000, and 18,000 rpm from right to left for each concentration) indicates DdrB is consistently pentameric. The plot represents the ratio for each condition between the observed mass of the protein complex (average value of 98.9 kDa) and the mass of DdrB monomer (20.8 kDa) against the average A279 nm reading of each gradient. B, fitting of the observed sedimentation equilibrium data (squares) against a model of a single species of pentamers (lines). The best fit is obtained with a mass ratio of 4.75. Results shown are from the most concentrated sample (24.12 μm) for the five tested speeds. C, gel filtration of the globular protein standards (▴) and DdrB protein (■) on a Sephacryl S-300 HR column. Apoferritin (443 kDa), β-amylase (200 kDa), bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa) were used to calibrate the column. The best-fit line was generated using the Kav of the standards against their molecular weight (MW) as represented by the equation. Native DdrB presented a Kav of 0.258, which corresponds to an apparent size of 133 kDa.