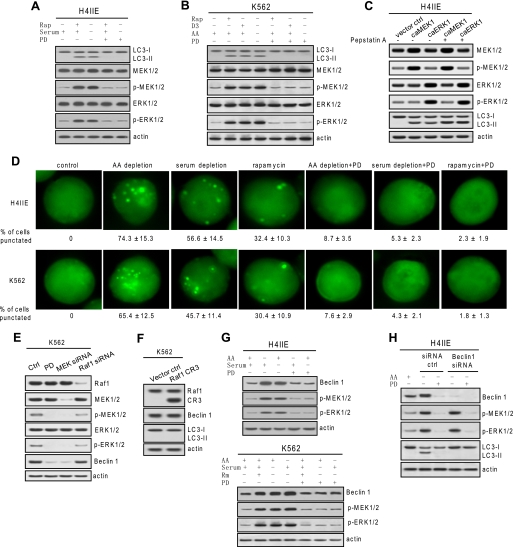
FIGURE 1.
Autophagic activity and Beclin 1 expression is MEK/ERK-dependent. A and B, autophagic response coincided with MEK/ERK signaling. H4IIE and K562 cells were treated with various autophagy stimuli (10 μm rapamycin (Rap) for 48 h, serum depletion for 48 h, amino acid (AA) depletion for 6 h, or 50 nm vitamin D3 (D3) for 72 h) with or without 2 μm PD98059 (PD). C, constitutive expression of active MEK or ERK triggered autophagic responses. H4IIE cells were transfected with constitutively active (ca) MEK or active ERK, and LC3 processing was determined in the presence of lysosomal protease inhibitor pepstatin A. D, fluorescence microscopy of GFP-LC3 transfectant cells undergoing the designated treatments. The percentage of cells with a punctate pattern is indicated as means ± S.D. (n = 3). E, basal MEK/ERK activity confers constitutive Beclin 1 expression. Depleting basal phospho (p)-MEK1/2 but not Raf1 by siRNA depleted basal Beclin 1. F, constitutive expression of CR3 domain of Raf1 did not trigger autophagic responses. G, autophagy stimuli-enhanced Beclin 1 was diminished by inhibition of MEK/ERK. H4IIE and K562 cells were starved for amino acids for 6 h or serum for 48 h or treated with 10 μm rapamycin (Rm) together with or without 2 μm PD98059. H, depleting Beclin 1 abolished autophagy in response to MEK/ERK activation. Beclin 1 knockdown H4IIE cells were starved for amino acids with or without 2 μm PD98059. Shown in this figure from A to G are representative Western blots or fluorescence microscopy of each of three independent experiments. ctrl, control.