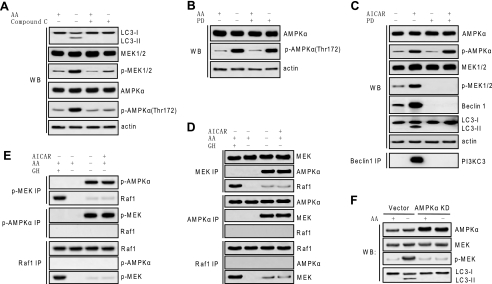
FIGURE 3.
MEK/ERK is positioned downstream of AMPK in autophagic signaling. A, inhibiting AMPK inhibited MEK activation in response to autophagy stimuli. H4IIE cells were starved for amino acids (AA) and treated with 10 μm compound C. B, inhibition of MEK did not inhibit AMPK activation in response to autophagy stimuli. H4IIE cells were starved for amino acids and treated with or without 2 μm PD98059 (PD), and AMPK activation was determined. C, up-regulation of Beclin 1 and induction of autophagy by AMPK activation was dependent on MEK. H4IIE cells were treated with 1 mm AICAR with or without 2 μm PD98059, and activation of AMPK and MEK, expression of Beclin 1, LC3 processing, and Beclin 1 binding to PI3KC3 were determined. D and E, MEK interacted with AMPK but not Raf1 in response to autophagy stimuli. H4IIE cells were starved for amino acids or treated with 1 mm AICAR, and physical interaction between MEK and AMPK, between Raf1 and MEK, or between AMPK and Raf1 was determined by using total (D) or phospho antibody (E). Growth hormone (GH) treatment served as a positive control for canonical Raf/MEK/ERK activation and interaction in H4IIE cells. F, specific inhibition on AMPK by kinase-dead AMPK crippled activation of MEK and autophagic activity in response to autophagy stimuli. H4IIE cells stably transfected with either rat AMPKα2 kinase-dead (KD) construct or vector control were starved for amino acids for 6 h. Expression of total AMPKα and total and phospho (p)-MEK and processing of LC3 were determined. Shown in this figure from A to F are the representative Western blots (WB) or co-immunoprecipitate (IP) blots of each of three independent experiments.